Carbene Formation or Reduction of the Diazo Functional Group? An Unexpected Solvent-Dependent Reactivity of Cyclic Diazo Imides
Abstract
The control of the reactivity of diazo compounds is commonly achieved by the choice of a suitable catalyst, e.g. via stabilization of singlet carbenes or radical intermediates. Herein, we report on the light-promoted reactivity of cyclic diazo imides with thiols, where the choice of solvent results in two fundamentally different reaction pathways. In dichloromethane (DCM), a carbene is formed initially and engages in a cascade C−H functionalization/thiolation reaction to deliver indane-fused pyrrolidines in good to excellent yields. When switching to acetonitrile solvent, the carbene pathway is shut down and an unusual reduction of the diazo compound occurs under otherwise identical reaction conditions, where the aryl thiol acts as reductant. A combined set of experimental and computational studies was carried out to obtain mechanistic understanding and to support that indane formation proceeds via the insertion of a triplet carbene, while the reduction of diazo imides proceeds via an electron transfer process.
Carbenes are divalent carbon-based reactive intermediates with two unpaired electrons and can take up two different spin states—the singlet or the triplet state.1, 2 The low-valent nature of carbenes makes these intermediates highly reactive and results in rapid undesired decomposition reactions3 and led to the long-standing paradigm that stabilization with metal complexes is required to attenuate the reactivity of carbenes4, 5 and to achieve efficient carbene transfer reactions.6 Lately, however, several groups disclosed that photochemical conditions and visible-light irradiation can be employed to achieve catalyst-free carbene transfer reactions,7, 8-10 which results from a specific substitution pattern on the carbene center for stabilization of the singlet state of carbenes to minimize side reactions. Such singlet carbenes then, for example undergo dearomatization and norcaradiene formation with benzene derivatives,9 or C−H functionalization with electron-rich N-heterocycles (Scheme 1a).10
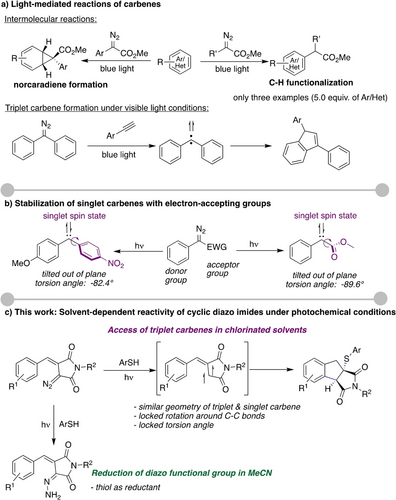
Control of the spin state of singlet and triplet carbenes. a) light-mediated reactions of carbenes. b) Stabilization of singlet carbenes with electron-accepting groups. c) Solvent-dependent reactivity of diazo imides.
Although the triplet carbene is of indispensable value to open up orthogonal reaction pathways to singlet carbenes, studies on their synthetic applications are less common.11, 12 One of the rare reports on the synthetic application of free triplet carbenes describes the photochemical decomposition of diphenyl diazomethane to triplet diphenyl carbene and the reaction with terminal alkynes (Scheme 1a). In this specific case, the triplet carbene was shown to be preferred over a singlet carbene, thereby resulting in the cascade reaction to give indenes.11 An alternative for such a photochemical approach lies within the use of a photosensitizer and triplet sensitization, yet being limited mainly to ethyl diazoacetate.12 Despite this progress, the access of free triplet carbenes under photochemical, catalyst-free conditions and the achievement of efficient carbene transfer reactions of these intermediates remains an intricate synthesis challenge and is in high demand to further explore the reactivity of this diradical species in organic synthesis.
To achieve this goal, we considered that the C−C bond of the carbene center to one of its substituents undergoes a significant perturbation in the case of preferential formation of singlet carbenes (Scheme 1b). It is suggestive that the rotation of the electron-deficient group results in a conformation that aids in the stabilization of the singlet spin state. Specifically, it was reported by Sander,13 Davies,14 and Koenigs11 that one substituent adopts an almost perpendicular conformation by out-of-plane rotation in the case when a singlet diaryl carbene is preferentially formed over a triplet carbene.
We contemplated that the hindrance of this rotation may result in a favorable formation of a triplet carbene by destabilization of the singlet spin state. This in turn could lead to a selective reaction on the triplet spin surface. We, therefore, turned our attention towards cyclic diazo compounds, where the cyclic nature prevents free rotation and should thus lead to the favorable formation of a triplet carbene. Herein, we report our findings on this endeavor and discuss the photochemical reaction of a cyclic diazo imide with aryl thiols. We observed an unusually high solvent dependency, where an intramolecular C−H insertion reaction, followed by the addition of the thiol is observed in most solvents (Scheme 1c). When changing the solvent to acetonitrile, however, a surprising, unexpected reduction of the diazo functional group to a hydrazone was observed, where the thiol acts as a reducing agent.
We therefore examined the reaction of the cyclic diazo imide 1 a with p-thiocresol (2 a) at 365 nm in dichloromethane. The reaction was rapidly completed within 10 minutes and product 3 a was obtained as a single diastereomer with a good yield of 57 %. Studies on the influence of reaction stoichiometry and additives are shown in Table S2 of ESI15 and quickly led to the optimized conditions (Scheme 2). An unexpected and surprising observation was made when using acetonitrile as a solvent. Under otherwise identical conditions, diazo imide 1 a was rapidly consumed, and hydrazone 4 a was obtained in good yields within only three minutes of reaction time. As the by-product of this reaction, we observed the formation of the disulfide, which suggests that thiol 2 a reacts as a reducing agent in this reaction (Scheme 2).
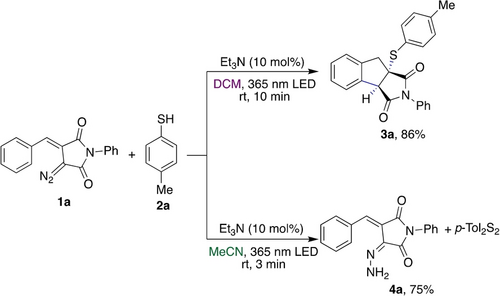
Solvent-dependent reactivity of cyclic diazo imides.
We next performed experimental and theoretical studies for insight and rationalization of the reaction outcome. No reaction occurred in the dark, which indicates the necessity of light to initiate the reaction. When performing the reaction in the absence of thiol or with a disulfide under the standard reaction conditions in DCM solvent, the anticipated intermediate 5 a was obtained in 48 % or 39 % yield, respectively (Scheme 3a). The crude 1H NMR spectra recorded in CDCl3 at an interval of 2-minutes showed that intermediate 5 a is formed initially and then the addition of thiol 2 a takes place to provide the desired product.15 To assess if 5 a is a viable intermediate in this reaction, we next performed the reaction of isolated intermediate 5 a with 2 a under light and dark conditions. Under irradiation, the desired product 3 a was formed rapidly (<5 minutes) in 94 % yield; however, a rather slow reaction was observed in the dark with a lower yield of 3 a. These results indicate that photoexcitation of 5 a is critical for the C−S bond formation. Further, the deuterium labelling experiments also indicate that photoexcitation of diazo imide and indane formation is a key step for this reaction (Scheme 3c, Scheme S6). When performing the model reaction in the presence of 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO, 2.0 equiv) as a radical trapping agent, the desired reaction was completely quenched. Along with the two adducts of imide and thiol with TEMPO, an adduct involving carbene+thiyl radical+TEMPO was observed in High Resolution Mass Spectrometry (HRMS, Scheme 3d, Scheme S7), which indicates the involvement of a diradical species. In addition, when the same reaction was performed in the absence of p-thiocresol, 5 a was not formed, however an adduct 2 x TEMPO + carbene was seen in HRMS (Scheme S7).
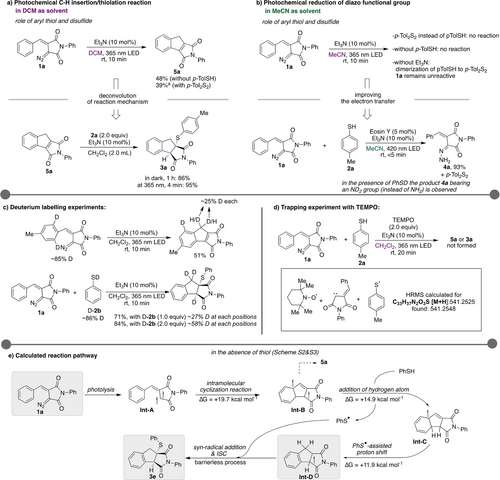
Mechanistic studies on the photochemical reaction of diazo imides. a) Photochemical C−H insertion/thiolation reaction. b) Photochemical reduction of diazo functional group. c) Deuterium labelling experiments. d) Trapping experiments with TEMPO. e) Calculated reaction pathway. a NMR yield.
We next studied control reactions for the reduction of the diazo imide in acetonitrile. In the absence of thiol 2 a or with a disulfide, the reduction product 4 a was not obtained. This finding supports the role of the thiol as a reducing agent (Scheme 3b). In the absence of triethylamine, diazo imide 1 a remains untouched in acetonitrile, however, the formation of disulfide was observed. The deuterium labeling experiment with deuterated thiophenol shows that deuterium is incorporated at the terminal nitrogen of the hydrazone (see Scheme S6). We, therefore, consider the formation of a reactant complex and an electron transfer as a viable reaction pathway and that acetonitrile as solvent facilitates this process. Building on this assumption, we studied the reduction of diazo imide 1 a in the presence of Eosin Y, which can easily undergo single electron transfer reactions.16 To our delight, the reduction of diazo imide 1 a to hydrazone 4 a occurred in excellent yield, when using 5 mol % Eosin Y as a photosensitizer at 420 nm, while slightly lower yields were obtained upon using longer wavelengths (Scheme 3b, Scheme S3).
Based on these control experiments, we next embarked on computational studies (Scheme 3e). Due to the good efficiency of the reaction in the absence of triethyl amine and to reduce the computational costs, we excluded NEt3 in our calculations. We assume that NEt3 reduces energy barriers of transition states and/or stabilizes intermediates. Based on our computational studies, we suggest the formation of a triplet carbene intermediate Int-A via initial photoexcitation of diazo imide followed by extrusion of nitrogen gas with an energy barrier of +10.0 kcal mol−1 (Scheme S1) as the initial reaction step. Calculations on the formation of a singlet carbene intermediate indicate that such a pathway is not feasible due to a) the high energy barrier for the formation of the singlet carbene (+32.5 kcal mol-1 and b) the triplet carbene is favored by 2.1 kcal mol−1 over a singlet carbene. In the next step, the intramolecular cyclization into the neighboring C(sp2)−H bond occurs with an activation energy of +19.7 kcal mol−1, leading to the formation of Int-B. The latter reacts with PhSH with an activation free energy of +14.9 kcal mol−1 to give radical intermediate Int-C and PhS radical. Following a sequence of PhS-assisted hydrogen shifts intermediate Int-D is furnished. In the last step of the reaction mechanism, a thiyl radical (PhS⋅) adds to intermediate Int-D in a barrierless process and forms the desired product after inter system crossing (ISC). The higher efficiency of the reaction in the presence of NEt3 can be explained by a more efficient formation of intermediate Int-F in the presence of a base (Schemes S2, S3 and S4).
With the optimized conditions in hand, we then explored the substrate scope by varying the substitution on the aryl ring of the thiophenols and diazo compounds (Scheme 4). At the outset, a wide range of electron-rich, -deficient and -neutral aryl thiols were studied. Most of these aryl thiols are well tolerated in this transformation without any prominent impact on yield and diastereoselectivity of the products 3 a–m even at 1.0 mmol reaction scale. Also, 2-naphthyl thiol underwent smooth transformation providing the desired product 3 n in 66 % yield. A heteroaromatic thiol, i.e. 2-thiophene thiol, worked well to provide the corresponding product 3 o in 53 % yield.
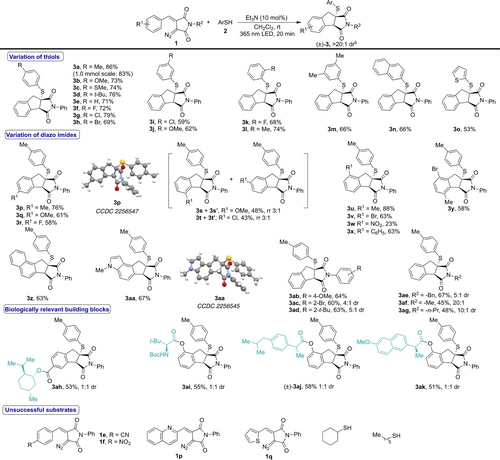
Substrate scope for C−H functionalization/thiolation reaction. The reactions were carried out using 1 (0.2 mmol), 2 (2.0 equiv.) and Et3N (10 mol %) in dry dichloromethane (4.0 mL). Yield refers to the isolated yield after column chromatography. Unless specified, a single diastereomer (>20 : 1 dr) of the product was observed.
The electronic properties of the substituent (R1) on the aryl ring of the diazo imide 1 have a moderate effect on the yields of the corresponding products 3 p–3 y. In the case of meta-substituted diazo imides, the major products 3 s and 3 t were obtained with a ratio of 3 : 1 with regards to the respective regioisomers 3 s′ and 3 t′. In addition, diazo compounds bearing a naphthyl or an indole ring also worked well, providing the desired products 3 z and 3 aa as single diastereomers. Additionally, different N-aryl groups on the diazo compound 1 were studied and a smooth transformation under the standard conditions was observed to give the corresponding products 3 ab–ad. In the case of ortho-substituted N-aryl groups a diastereomeric ratio of 4 : 1 and 5 : 1 was observed for the products 3 ac and 3 ad, respectively. Similarly, N-alkyl groups on substrate 1 were tolerated to provide the corresponding products 3 ae–ag in good yield and a diastereomeric ratio of up to 20 : 1. The structures and relative configuration of the products 3 a–3 ag were established based on X-ray crystal structures17 of 3 p and 3 aa. The reactions proceeded smoothly with the diazo compounds bearing menthol, an amino acid, or drug molecules as the side chain (Scheme 4).
The limitations of the present method lie within the use of aliphatic thiols, which were found not compatible with this transformation probably due to the lesser stability of alkyl thiyl radicals. Similarly, strongly electron-withdrawing cyano- and nitro-groups on the C-4 position of the aryl ring of diazo imides (1 e and 1 f) or heteroaryl-derived diazo imides (1 p and 1 q) failed to provide the desired products due to the decomposition of the corresponding diazo imides.
Finally, we turned our attention to the unusual solvent dependency of this reaction and studied the generality of the reduction of diazo functional groups with thiophenol derivative 2 a under photochemical conditions using 5 mol % of Eosin Y (Scheme 5). In this reaction, the electronic properties of the substitution on the aryl ring had no apparent effect on the yield of the corresponding hydrazones 4 a–r. For example, strongly electron-withdrawing (NO2) and -donating groups (OMe), indolyl, thienyl and menthyl groups were well-tolerated in this transformation to afford the desired products in excellent yields.
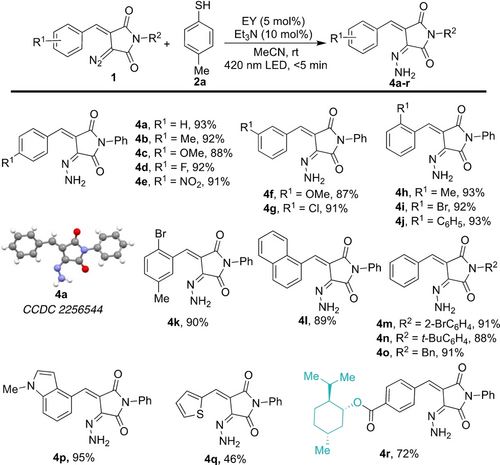
Substrate scope for reduction of diazo alkanes to hydrazones. The reactions were carried out using 1 (0.2 mmol), 2 a (3.0 equiv.), Et3N (10 mol %) and Eosin Y (5 mol %) in CH3CN (4.0 mL). Yield refers to the isolated yield after column chromatography.
Indane-fused pyrrolidines are an essential core of many biologically important compounds. For instance, indenopyrrole I is an integrin agonist, II is an antihypertensive agent (Scheme 6a).18 Most of the recent strategies for synthesizing such pyrrolidine-2,5-diones rely on transition metal-catalyzed C−H activation reactions.19-23 These strategies provide the corresponding products in excellent diastereoselectivity, however, the requirement of expensive metal catalysts, metal additives, high reaction temperature and the need of suitably placed directing groups are shortcomings of these methods. Therefore, the development of more sustainable metal-free approaches of indane-fused pyrrolidine-2,5-diones is in high demand. Hence, we studied synthetic applications of the product of C−H functionalization/thiolation reaction, by synthesizing indane-fused pyrrolidine 6 via LiAlH4-mediated reduction of the imide group of 3 a (Scheme 6b). Furthermore, sulfoxide 7 and sulfone 8 were also obtained in very good yields by oxidation of 3 a with meta-chloroperoxybenzoic acid (m-CPBA). The synthetic utility of hydrazone 4 a was also demonstrated via a diisobutylaluminium hydride (DIBAL) mediated chemoselective reduction to afford a hemiaminal 9 (Scheme 6c). This observation is in contrast to the DIBAL reduction of 3 a, which gave pyrrolidine 6.
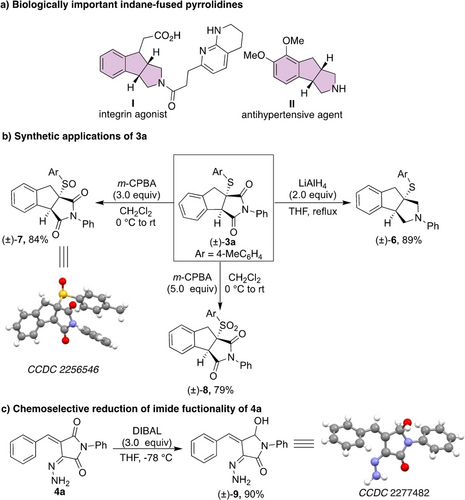
Biologically relevant indane-fused pyrrolidines and potential synthetic applications of indane-fused pyrrolidinone and hydrazone product.
In summary, we have disclosed an unusual solvent-dependent divergent reactivity of diazo imides with aryl thiols under photochemical conditions. In chlorinated solvents, a light-mediated metal-free regio- and diastereoselective synthesis of indane-fused pyrrolidine-2,5-diones was achieved via carbene-formation followed by C−H aryl insertion and thiol addition. Switching the solvent to acetonitrile led to the reduction of diazo imide functional group, where the aryl thiol acts as reductant. Both reaction pathways tolerate a wide range of aryl-substituted diazo compounds and thiophenols, delivering products in good to excellent yield and diastereoselectivity in the case of indane-fused pyrrolidine-2,5-dione synthesis. The developed method can be utilized for the functionalization of bioactive molecules and drugs, therefore providing a streamlined access towards indane-fused pyrrolidines.
Acknowledgments
P. C. acknowledges the Council of Scientific and Industrial Research (CSIR) India (File No.: 02(0422)/21/EMR-II) and Science & Engineering Research Board (SERB) India (File No.: CRG/2022/003212) for generous support. We also thank Central Instrumentation Facility SAPTARSHI-IIT Jammu for the instrumentation facility. Y. H. thanks UGC-India for the fellowships. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




