Palladium-Catalyzed Cascade Carbonylation to α,β-Unsaturated Piperidones via Selective Cleavage of Carbon–Carbon Triple Bonds
Dedicated to Professor Dr. Christian Bruneau on the occasion of his birthday and excellent work in organometallic catalysis
Abstract
A direct and selective synthesis of α,β-unsaturated piperidones by a new palladium-catalyzed cascade carbonylation is described. In the presented protocol, easily available propargylic alcohols react with aliphatic amines to provide a broad variety of interesting heterocycles. Key to the success of this transformation is a remarkable catalytic cleavage of the present carbon–carbon triple bond by using a specific catalyst with 2-diphenylphosphinopyridine as ligand and appropriate reaction conditions. Mechanistic studies and control experiments revealed branched unsaturated acid 11 as crucial intermediate.
Introduction
Compared with carbon–carbon bond-forming processes, the corresponding selective cleavage reactions are much less developed. Hence, this area is still considered as one of the holy grails in modern organic synthesis.1 While significant progress has been made in the past decades, specifically with C−C and C=C bonds,2, 3 the cleavage of carbon–carbon triple bonds is rare and remains a highly challenging subject4 owing to the extraordinarily large bond dissociation energy (>200 kcal mol−1).1m Apart from alkyne metathesis (Scheme 1 a),5 such transformations need stoichiometric amounts of special organometallic reagents, oxidants, etc.6 Indeed, only few catalytic methods are known which involve C≡C triple bond scissions.7 Remarkably, unlike these reported alkyne cleavage reactions, here we present a novel breaking/rearranging annulation reaction of alkynols which offers a step- and atom-economical route for the synthesis of α,β-unsaturated piperidones.
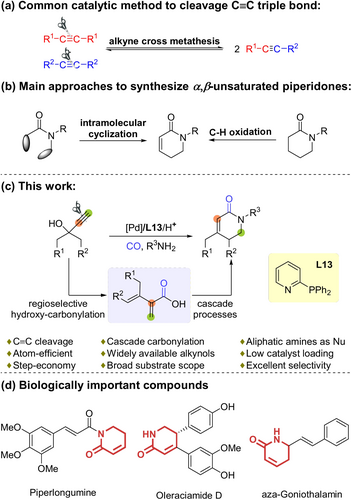
a) Summary of the main catalytic methods for C≡C cleavage. b) Summary of the main currently available synthetic methods for α,β-unsaturated piperidones. c) This work. d) Selected examples of biologically active molecules.
α,β-Unsaturated piperidones are part of many biologically important compounds. Thus, they are commonly used as building blocks of the preparation of natural compounds and to access new bio-active molecules.8 For example, Piperlongumine is a natural product reported to selectively kill cancer cells over non-malignant cells in vitro and in vivo.8a In addition, Oleraciamide D showed cytotoxicity against SH-SY5Y cells,8b while aza-Goniothalamin inhibited Ehrlich tumor growth (Scheme 1 d).8c Thus, several methodologies have been established towards their synthesis in recent years.9 Main approaches to this class of compounds include C−H oxidation reactions9a-9c and intramolecular cyclization reactions (Scheme 1 b).9d-9h Despite these significant advances, still limitations exist with respect to substrate scope and the use stoichiometric amounts of oxidants or additives.
As one of the most important methods for producing value-added carboxylic acid derivatives from unsaturated compounds, transition-metal-catalyzed carbonylation reactions have been extensively investigated and applied in academia and industry for several decades.10 In this respect, aminocarbonylation allows for atom-economic and convenient way to construct α,β-unsaturated piperidones. However, only few intramolecular amino-carbonylation reactions were reported, which exclusively worked for specific substrates and suffered from poor yields.11
Recently, we started to investigate the alkoxy- and aminocarbonylation of propargylic alcohols.12 During the course of this work, we discovered that α,β-unsaturated piperidones can be synthesized in a straightforward and general manner by a new palladium-catalyzed cascade carbonylation of alkynols and aliphatic amines through highly selective C≡C cleavage reaction (Scheme 1 c).
Results and Discussion
We started our investigations using the carbonylation of 1-ethynyl-1-cyclohexanol 1 a with benzylamine 2 a. Notably, this transformation is intrinsically challenging: First of all, benzyl amine and related aliphatic ones are generally not suitable for these carbonylations owing to the stronger basicity, which retards the generation of the catalytically active palladium hydrides.13 Obviously, alkynol such as 1 a is prone to produce many products such as the corresponding branched or linear α,β-unsaturated amides, lactones and acids. In Figure 1 and Scheme 2, selected carbonylated products, which might originate from this benchmark reaction are shown. To control the desired palladium-catalyzed aminocarbonylation reaction, a highly selective catalyst system is needed to balance the compatibility of reactant partners and the speed of individual elementary steps.14 In general, the choice of ligand is crucial for controlling both selectivity and activity.15 Thus, we tested different mono- and bidentate phosphine ligands (4 or 2 mol %, respectively) in the carbonylation of 1 a in the presence of 1 mol % PdBr2, HFIP (solvent), 40 bar CO, and 120 °C. For comparison, no reaction occurred without ligand or palladium present. Initially, some commonly used bisphosphine ligands L1–L4 were evaluated. While DPEphos L1, bis(2-dicyclohexyl-phosphinophenyl) ether L2, 1,2-bis((di-tert-butylphosphan-yl)methyl)benzene L3 did not exhibit any catalytic activity, L4 with 2-pyridyl-tert-butylphosphino groups gave the linear α,β,γ,δ-unsaturated amide 4 as main product. In the presence of monophosphine ligands L5–L8 with different backbones and chelating units, no conversion was observed. Surprisingly, using the imidazole-based ligand L9, product 3 aa was afforded in 40 % yield, together with lactone 5 as side product (11 % yield). Clearly, 3 aa cannot be formed by a traditional alkyne carbonylation–cyclization sequence. Next, other monophosphine ligands L10–L12 were tested and 3 aa was obtained in similar yields (36–42 %), together with lactone 5 as major side product (5–15 % yield). Gratifyingly, diphenyl(2-pyridyl)-phosphine L13, which was introduced by Drent et al.,16 gave an improved yield of 3 aa (60 %). By variation of critical reaction parameters (catalyst precursor, acidic co-catalyst, temperature, pressure, etc.) in the presence of L13, the yield of 3 aa reached 78 % at a low catalyst loading (0.2 mol % Pd) (see Supporting information, Tables S1–S5).
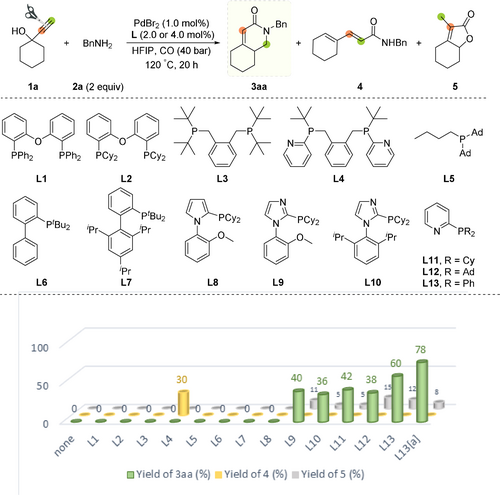
Pd-catalyzed carbonylation of 1-ethynyl-1-cyclohexanol 1 a with benzylamine 2 a: Influence of phosphine ligands. Reaction conditions: 1 a (0.5 mmol), 2 a (1.0 mmol), PdBr2 (1.0 mol %), bisphosphine ligand (2.0 mol %) or monophosphine ligand (4.0 mol %), CO (40 atm), HFIP (2.0 mL), 20 h at 120 °C. The yields of products were determined by GC analysis with mesitylene as internal standard. [a] Pd(dba)2 (0.2 mol %), L13 (0.8 mol %), TfOH (10 mol %).
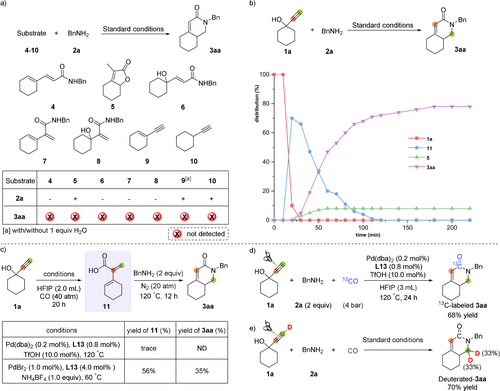
Standard conditions: Pd(dba)2 (0.2 mol %), L13 (0.8 mol %), TfOH (10 mol %), HFIP (2.0 mL), CO (40 atm), 120 °C, 20 h. 1 a or 4–10 (0.5 mmol), benzylamine 2 a (1.0 mmol). The yields of products were determined by crude 1H NMR analysis using dibromomethane as the internal standard. a) Control experiments with 4–10. b) Kinetic monitoring of intermediates over time: The X-axis represents reaction time, and the Y-axis represents intermediate distribution. c) Control experiments with 11. d) Reaction with labeled 13CO. e) Reaction with deuterated 1 a [a] Pd(dba)2 (0.2 mol %), L13 (0.8 mol %), TfOH (10 mol %), 1 a (0.5 mmol), benzylamine 2 a (1.0 mmol), H2O (0.5 mmol), HFIP (2.0 mL), CO (40 atm), 120 °C, 20 h.
To gain insights into the reaction mechanism and the formation of the unexpected product 3 aa, several control experiments were conducted (Scheme 2 a–d). First, the carbonylation of 1-ethynylcyclohexene 9 (with or without 1 equiv water) was examined under the standard conditions. However, formation of 3 aa was not observed, showing the reaction did not proceed through the equilibrium of alkynol 1 a with 1-ethynylcyclohexene 9. Instead, 7 was obtained in 33–60 % yield (see Supporting information, Scheme S1).
Using cyclohexylacetylene 10 under identical conditions failed to give any carbonylation product, demonstrating the crucial role of the hydroxyl group in 1 a. Next, all the possible compounds (4–8) generated in the novel carbonylation sequence were prepared independently, isolated and examined to ascertain the intermediates leading to the formation of 3 aa. Astonishingly, reaction of amides 4, 6, 7, 8 and lactone 5 with CO under standard conditions provided in no case 3 aa, thus excluding the possibility that these compounds function as intermediates towards 3 aa (Scheme 2 a).
As another attempt to identify key intermediates, the kinetic progress of the reaction between alkynol 1 a and benzylamine 2 a was examined under the optimal conditions. As shown in Scheme 2 b, 1 a was initially converted into 2-(cyclohex-1-en-1-yl)acrylic acid 11. Then, 3 aa was generated along with the consumption of the reaction intermediate 11. Starting material 1 a was fully converted after 30 min and formation of the branched α,β,γ,δ-unsaturated acid 11 achieved a maximum yield of 70 % after 20 min. In the following three hours, the yield of desired product 3 aa increased steadily and reached 78 % yield after 180 min. Notably, the side-product lactone 5 started to be generated after 30 min and kept in a small amount (8 % yield).
Next, control experiments were performed by using acid 11 as starting material (Scheme 2 c). At this point it should be noted that acid 11 has not been isolated before and proved to be a highly reactive compound, which easily underwent oligomerization/polymerization; thus, only traces of 11 were detected in the control reaction without benzylamine 2 a. However, the desired intermediate could be prepared in situ in 56 % GC yield in the presence of NH4BF4 at 60 °C. Finally, the target compound α,β-unsaturated piperidone 3 aa was produced in 35 % yield after addition of benzylamine 2 a into the system and heating at 120 °C at N2 atmosphere. This reaction sequence confirmed that acid 11 is indeed a reaction intermediate in the overall process.
To clarify the origin of the acyl group in piperidone 3 aa, the reaction was carried out under 4 atm 13CO atmosphere (Scheme 2 d). Thus, 13C-labeled 3 aa was synthesized smoothly in 68 % yield, which revealed that carbon monoxide forms the CO moiety of 3 aa. When deuterated alkynol 1 a was tested under optimized conditions, the D atom ended up on the carbon in α-position to the nitrogen atom (Scheme 2 e).
Based on all these results and previous mechanistic studies of Pd-catalyzed carbonylations,15 we suggest the following mechanism of this novel palladium-catalyzed cascade carbonylation of alkynols (Scheme 3). Initially, the catalytic cycle starts from the cationic palladium hydride species B, which is generated from Pd0 phosphine complexes A after protonation in the presence of acid. Subsequently, the carbon–carbon triple bond of alkynol 1 a coordinates to the metal center to form complex C, followed by Pd-H insertion into the alkyne, which affords the branched alkenyl-Pd intermediate D regioselectively. The regioselectivity of this process is controlled by the 2-pyridyldiphenylphosphine ligand. Then, Pd complex D transforms into the corresponding acyl Pd species E through a facile CO insertion process. After dehydration of hydroxy group and hydrolysis with water as nucleophile, intermediate E leads to 2-(cyclohex-1-en-1-yl)acrylic acid 11 and regenerates the palladium hydride species B. After cycle I, the π-allyl-palladium intermediate F is formed selectively by Pd-H insertion into the cyclic olefinic bond of 11. This intermediate undergoes a fast equilibrium with the corresponding σ-palladium complexes G. Next, amination occurs regioselectively at the less hindered position leading to amino acid H. We hypothesize that H is highly reactive, which gives the unsaturated β-lactam I upon cyclization. It should be noted that the developed catalyst system could be crucial as the ligand might facilitate this key step by acting as a proton shuttle. Owing to the ring-strain in the resulting 4-membered ring compound, I is prone to relief the energy by isomerization to J. We assume through a π-allyl-palladium intermediate K,17 the more stable ring expanded product 3 aa is formed.
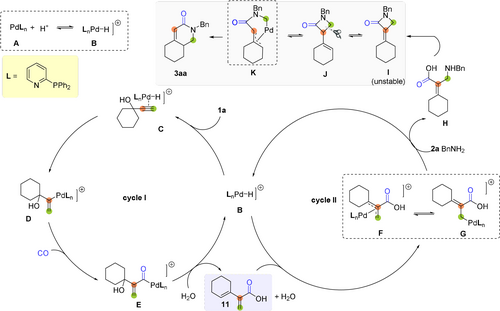
Proposed catalytic cycle.
With optimized conditions established for the model reaction, we examined the generality of the cascade carbonylation process. Actually, this novel transformation is observed with a variety of propargylic alcohols. As shown in Table 1 a, diverse α,β-unsaturated piperidones can be conveniently prepared in the presence of the optimal catalytic system. Simple aliphatic and aromatic substituents (tert-butyl, phenyl) at the 4-position of cyclohexyl group were well tolerated to furnish 3 ba (dr=3/1) and 3 ca (dr=2.4/1) in good yields (51 % and 53 %, respectively). An array of carbocyclic ring systems (five-, eight-, and twelve-membered) reacted smoothly, providing the corresponding α,β-unsaturated piperidones 3 da–3 fa in 58–82 % yields. Similarly, substrates 1 g–1 i containing heteroatoms (oxygen, sulfur, nitrogen) were amenable to this approach, giving rise to 3 ga–3 ia in 46–63 % yields with an increased catalyst loading (1 mol %). Furthermore, non-cyclic alkynols 1 j–1 m bearing different alkyl and benzyl groups afforded the corresponding desired products 3 ja–3 ma in 58–90 % yields. When 3-methylhept-1-yn-3-ol 1 n was subjected to the optimized conditions, the corresponding carbonylation product was obtained in 75 % yield with 1.5/1 regioselectivity (3 n′a/3 na). On the other hand, starting from α-alkyl-aryl-substituted alkynols 1 o–1 r, carbonylation proceeded successfully and delivered 3 oa–3 ra in 25–74 % yields.

- [a] Standard reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), Pd(dba)2 (0.2 mol %), L13 (0.8 mol %), TfOH (10.0 mol %), CO (40 atm), HFIP (2.0 mL), 20 h at 120 °C. Isolated yields were given within the parentheses. The NMR yields (values before the parentheses) were determined by crude 1H NMR analysis using dibromomethane as the internal standard. [b] 1 n (0.1 mmol), 2 a (0.2 mmol), Pd(dba)2 (1.0 mol %), L13 (4.0 mol %), TfOH (20.0 mol %). [c] 1 a (0.25 mmol), 2 a (0.5 mmol), Pd(dba)2 (0.4 mol %), L13 (1.6 mol %), TfOH (20.0 mol %). [d] Ellipsoids correspond to 30 % probability. Only one of the two molecules of the asymmetric unit is depicted; see Figure S1 in Supporting Information 1.4.
Next, we evaluated the general scope of this new three component process with respect to the reactivity of alkylamines. As shown in Table 1 b, various benzylamines with electron-neutral, electron-deficient, and electron-rich substituents proved to be useful. Besides, substituents in para-, meta-, and ortho-position of the phenyl ring had no significant influence on the catalysis. Thus, 3 ab–3 ai were produced in 44–80 % yields. In addition, other primary alkylamines (2 j–2 m) including sterically crowded ones (2 n, 2 o) reacted smoothly and gave the corresponding piperidones 3 aj–3 ao in 30–67 % yields. Notably, thienyl- and pyridyl- substituted amines 2 p, 2 q were well tolerated, affording 3 ap and 3 aq in 51 % and 38 % yield, respectively. It is worth mentioning that functionalized amine with nonprotected hydroxy group can be transformed into the desired piperidone 3 ar in 65 % yield with excellent selectivity.
Finally, selected alkylamines 2 s–2 u containing methoxy, allyl, and homoallyl substituents reacted well to give the desired products 3 s–3 u in 34–77 % yields, which provides easy access to diverse α,β-unsaturated piperidone derivatives. To be noted, geranylamine 2 v and (−)-cis-myrtanylamine 2 w led to the desired products 3 av and 3 aw (dr=1/1) in 81 % and 51 % yield, respectively, highlighting the substrate scope of this protocol.
Conclusion
While studying the palladium-catalyzed functionalization of alkynols, we observed a novel catalytic C≡C cleavage reaction, which provides the basis for a unique redox-neutral cascade carbonylation process. Based on control experiments and mechanistic studies, here the unfavorable C≡C bond cleavage step takes place under relatively mild conditions. Key for this effective step is the formation of a high energy intermediate (unsaturated β-lactam), which releases the ring strain by C−C migration to make the overall reaction thermodynamically feasible. From a synthetic standpoint, this new transformation presents a practical strategy for the synthesis of α,β-unsaturated piperidones utilizing easily available propargylic alcohols and aliphatic amines directly as robust surrogates. Under optimal conditions, diverse α,β-unsaturated piperidones were achieved in a fast and step-economic way, without need for isolation of intermediates. In fact, 40 out of 41 here prepared molecules have not been described before to the best of our knowledge. We believe this methodology also provides inspiration for conceptually new synthetic approaches complementing conventional organic synthesis.
Acknowledgements
This work is supported by the state of Mecklenburg-Western Pommerania and the BMBF (Bundesministerium für Bildung und Forschung) in Germany. We thank the analytical team of LIKAT, and Dr. Christoph Kubis for the support of 13CO. F.Y. thanks the National Natural Science Foundation of China (No. 21801056) for financial support. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




