Searching for Monomeric Nickel Tetrafluoride: Unravelling Infrared Matrix Isolation Spectra of Higher Nickel Fluorides
Dedicated to Prof. Dieter Lentz on the occasion of his 70th birthday
Abstract
Binary transition metal fluorides are textbook examples combining complex electronic features with most fundamental molecular structures. High-valent nickel fluorides are among the strongest known fluorinating and oxidizing agents, but there is a lack of experimental structural and spectroscopic investigations on molecular NiF3 or NiF4. Apart from their demanding synthesis, also their quantum-chemical description is difficult due to their open shell nature and low-lying excited electronic states. Distorted tetrahedral NiF4 (D2d) and trigonal planar NiF3 (D3h) molecules were produced by thermal evaporation and laser ablation of nickel atoms in a fluorine/noble gas mixture and spectroscopically identified by a joint matrix-isolation and quantum-chemical study. Their vibrational band positions provide detailed insights into their molecular structures.
Binary fluorides of the late 1st row transition metals (TM) are among the most efficient oxidative fluorinating agents, especially for the production of perfluorinated organic compounds.1 CoF3 is of industrial importance in the Flutec or Fowler processes,2 and higher nickel fluorides are considered to be the active fluorinating agents in the electrochemical fluorination of organic substrates (Simons process).3 Despite numerous attempts to elucidate this latter process the active species are still unknown.3, 4
Clearly, these particular properties are related to the peculiar electronic structure of these late TM fluorides, such as high ionization energies, the occupation of M−F antibonding molecular orbitals, and the lack of the so-called “primogenic repulsion”, caused by the absence of radial nodes in the 3d valence orbitals.5 Nickel is one of the most electronegative metallic elements.6 This implies a considerable covalent character of the Ni−F bonds, which increases with the oxidation state of nickel.7, 8 Particularly for high-valent 3d TM complexes the similar radial extent of the 3d valence and the 3p core shell leads to repulsion between core 3p and ligand valence electrons, which ultimately results in weakened metal-ligand bonds,5 a weak ligand-field, and the presence of several low-lying excited electronic (spin) states.9 Taken together, these circumstances represent a challenge for quantum-chemical predictions and a major impediment for the spectral analysis of the still elusive high-valent molecular nickel fluorides.
In addition to the trifluorides, the tetrafluorides of the 1st row TM are particularly interesting. Solid MnF4 releases elemental fluorine at temperatures >170 °C.10 It can be used for storage and purification of elemental fluorine.2 In contrast to solid MnF4 and NiF4, solid FeF4 or solid CoF4 are unknown, while the monomeric FeF4 and CoF4 species have been investigated by high-temperature vapor-phase mass spectrometry and cryogenic matrix-isolation spectroscopy.11, 12, 13
Solid NiF4 is one of the strongest known fluorinating oxidizing agents,2 but it is poorly characterized spectroscopically and even its solid-state structure is unknown. We have not found any report on molecular or gaseous NiF4.4 Fluorination of solid NiF2 provides thermally unstable higher nickel fluorides such as Ni2F5 (NiII3NiIVF10)14 or R-NiF3 (NiIINiVIF6),15 and neutral NiF4 is claimed to be formed at about −60 °C by treating [NiF6]2− salts in anhydrous HF with strong Lewis acids such as AsF5, SbF5, or BF3.16 NiF4 releases F2 above −55 °C and forms NiF3, which again releases fluorine above 20 °C to form NiF2.17 As far as we know molecular NiF3 has not yet been studied spectroscopically, but it has attracted the interest of a theoretical study because of its low-lying electronically degenerate excited states, which are prone to Jahn–Teller (JT) effects.18
We carried out spectroscopic investigations on molecular NiF3 and NiF4 for the first time. Different methods were applied to produce these species which were subsequently isolated in cryogenic rare-gas matrices. First, nickel atoms were vaporized by heating a nickel wire and allowed to react with elemental fluorine diluted in argon prior to deposition on a matrix support at 10 K. Vaporization of atomic nickel was also achieved by laser ablation using the 1064 nm fundamental of a Nd:YAG laser focused onto a rotating nickel target and trapping the products at 6–15 K. The laser ablation process is associated with a hot plasma plume and a bright broad-band radiation, where excited nickel atoms can react with elemental fluorine and atomic fluorine radicals seeded in excess of noble gases (neon or argon). This method is particularly useful for the generation of highly fluorinated species.13, 19 For example, laser ablation from a metallic cobalt target in a fluorine/argon gas mixture clearly produces not only molecular CoF, CoF2 and CoF3 but also CoF4 (for experimental details see the Supporting Information), which could be detected using its known IR band in solid argon reported in a previous study,12 in which CoF4 was obtained at 650 K from solid mixtures of CoF3 and TbF4 as atomic fluorine source in a perfluorinated nickel effusion cell.12 The wavenumber ordering of the νCo-F modes is CoF4 > CoF3 > CoF2 ≫ CoF, see the Supporting Information.
Thermally evaporated Ni atoms react with F2 on deposition (Figure 1) to yield limited amounts of NiF2 at 779.4 cm−1[20] and a broad band at 625.8 cm−1 assigned to molecular NiF on the basis of the gas-phase IR band of 58NiF (2Π3/2) at 634.7 cm−1,21 and our computed value of 639.1 cm−1 at the CCSD(T)/AVT(Q)Z-DK level (Table S4.2). UV and broadband photolysis, associated with the formation of F radicals, resulted in the dramatic growth of the bands due to NiF2 (Figure 1 (a,d)), and two additional sets of overlapping bands close to 800 cm−1. Like the NiF2 bands the well resolved 58, 60Ni isotopic pattern on the 800 cm−1 bands is consistent with linear NiF2 units (Table S3.1).22 These new bands were assigned to matrix sites of NiF2 in F2/Ar matrices (see the Supporting Information).
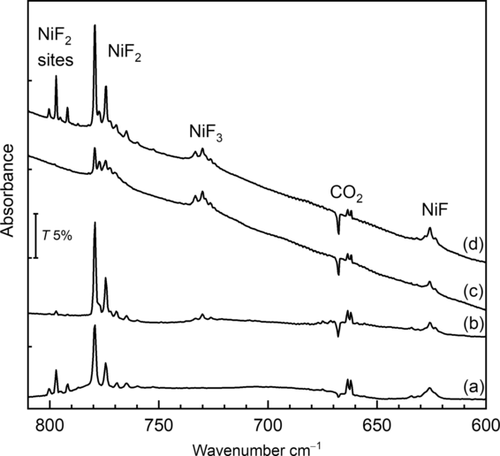
IR spectra of reaction products from thermally evaporated Ni atoms in 0.5 %F2/Ar matrices: a) after deposition and broadband photolysis, b) after annealing to 25 K, c) after deposition and annealing to 25 K, d) after broadband photolysis.
On annealing (Figure 1 (b,c)), the bands due to NiF2 increased while those close to 800 cm−1 decreased, and weak bands at 735–724 cm−1 appeared. These latter bands are assigned to the asymmetric stretch of molecular NiF3, trapped in different matrix sites. CCSD(T) calculations predict a 4A2′ ground state with D3h structure for molecular NiF3 (Scheme 1, Table S6.3) and a single infrared active E′ stretching fundamental about 40 cm−1 below that of NiF2 (Table S6.4).
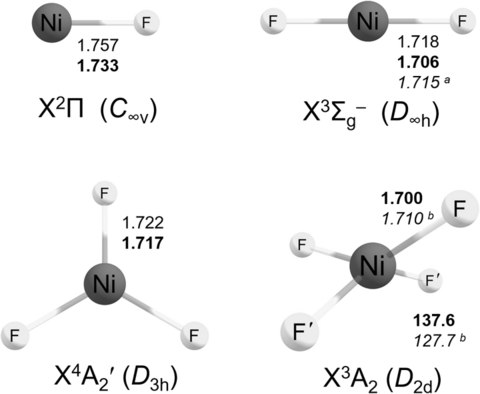
Structures of NiFn (n=1–4) species. Values calculated at the RCCSD(T)/AVTZ(NREL) and the RCCSD(T)/AVTZ-DK (in bold) levels of theory. [a] Experimental value from a gas phase electron diffraction study.23 [b] CASPT2/AVTZ-DK.
Subsequent nickel laser ablation experiments fully corroborate the assignment of NiF3 in solid argon (Figure 2). Due to the formation of considerably larger amounts of atomic fluorine radicals in the laser ablation experiment, the yield of NiF3 increased and further rose by annealing the deposit to 20 K (Figure 2). The NiF3 region is even more congested in the laser ablation experiment due the presence of different matrix sites. UV radiation of λ=273 nm (LED) depletes the NiF3 bands (band points upwards in Figure S4b) and forms NiF2, while λ=193 nm laser radiation destroyed both, NiF2 and NiF3 (Figure S4d). More interestingly, in these experiments a new band at 749.1 cm−1 with a distinct Δν(58/60Ni) isotope splitting of 5.1 cm−1 occurred (Figure 2). This band was very difficult to detect in the thermal evaporation experiment due to its much lower intensity. The carrier of this band appears to be formed by fluorination of NiF3, since it can only be observed when a sufficient amount of NiF3 is available and its intensity grows along with that of the NiF3 band, i.e. by annealing and with higher fluorine concentrations of the F2/Ar mixture (Figure S5). Based on this behavior, the new band is assigned to the strongest IR band of molecular NiF4. For a tetrahedral tetrafluoride only a single infrared-active Ni−F stretching mode is expected, however, for the d6 electronic configuration of NiF4 a Jahn–Teller distorted D2d structure is predicted at all levels of theory (Figure S7.1).
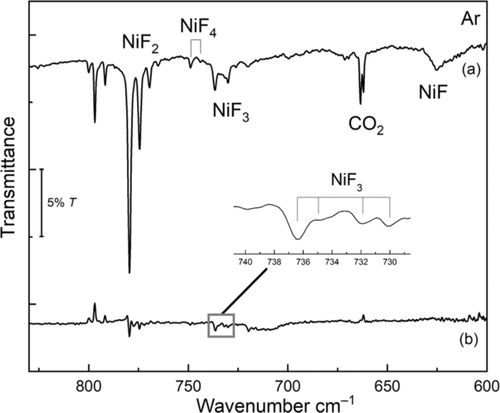
IR spectrum of the reaction products of laser-ablated Ni with F2 (0.5 %) seeded in excess argon: a) co-deposited for 60 min at 15 K, b) difference spectrum after annealing to 20 K.
We are not aware of previous high-level ab initio calculations for molecular NiF4. We carried out quantum-chemical investigations of various energetically low-lying electronic states of NiF4, especially of the lowest-energy triplet (3A2) and quintet (5B1, 5A1) states of D2d symmetry (Table S3.4). For the triplet ground state (Scheme 1) a strong IR band of E symmetry is predicted for NiF4 about 11 cm−1 blue-shifted from the E′ band of NiF3 at the CCSD(T)/AVTZ-DK level (Table 1), which is in excellent agreement with the observation. The much weaker predicted B2 band of NiF4 (Tables S7.2, S7.4, S7.6) was not detected. All four nickel fluorides are also observed in solid neon matrices (Tables 1, S3.3).
Species |
Ground State |
Sym. |
calcd[a] |
Exp. (Ne Matrix) |
Exp. (Ar Matrix) |
||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
58Ni |
60Ni |
58Ni |
60Ni |
58Ni |
60Ni |
NiF |
2Π |
C∞v |
NR[b] |
612.98 (Σ+) |
610.46 |
646.2 |
643.5 |
625.8 (broad) |
|
DK[b] |
639.13 (Σ+) |
636.50 |
|||||||
NiF2 |
3Σg− |
D∞h |
NR[c] |
806.42 (Σu+) |
801.09 |
800.1 |
794.9 |
779.5 |
774.4 |
DK[c] |
819.17 (Σu+) |
813.75 |
|||||||
NR[b] |
810.25 (Σu+) |
804.89 |
|||||||
DK[b] |
824.62 (Σu+) |
819.17 |
|||||||
NiF3 |
4A2′ |
D3h |
NR[c] |
762.95 (E′) |
758.67 |
743.8 |
740.0 |
736.5 |
731.7 |
DK[c] |
767.83 (E′) |
763.54 |
|||||||
NiF4 |
3A2 |
D2d |
NR[c] |
767.24 (E) |
762.56 |
762.3 |
757.2 |
749.1 |
744.0 |
DK[c] |
778.50 (E) |
773.71 |
|||||||
- [a] Non-relativistic (NR) and with scalar-relativistic Douglas-Kroll-Hess (DK) Hamiltonian; [b] RCCSD(T)/AVQZ; [c] RCCSD(T)/AVTZ.
A comparison of experimental M−F stretching bands of high-valent first-row metal fluorides is particularly revealing. The molecular trifluorides of M = Fe, Co, and Ni all adopt D3h structures, and their stretching fundamentals were found in Ne matrices, in a very narrow range, at 743.6 cm−1 (Fe),13 748.2 cm−1 (Co), and 743.8 cm−1 (Ni, Table 1). For a series of molecules with similar structures one expects a strong correlation between stretching frequencies and bond lengths, a trend that also applies to the bond length of CoF3 (1.722 Å) and NiF3 (1.722 Å, Table S3.5) obtained at the CCSD(T)/AVTZ level. A similar frequency-bond length correlation (Table S3.5) is expected for the stretching vibrations of the tetrafluorides MF4 of Fe to Ni observed in solid argon, which also appear in a narrow spectral range (757 cm−1 (M = Fe),13 767.8 (Co),12 749.1 (Ni)). However, this correlation is less accurate due to the Jahn–Teller deformed D2d molecular structures of FeF4 and NiF4 and due to stronger matrix interactions in solid argon.
Apart from these similarities, the higher nickel fluorides are strikingly different from their third-row predecessors. The rule of thumb that higher fluorides have shorter and stronger M−F bonds that applies to ionic metal fluorides does not apply to the higher nickel fluorides. While CCSD(T)/AVTZ calculations predict a successive M−F bond shortening for the ionic iron fluorides FeFn, with n=1–4,13 and, albeit significantly weakened, also for CoFn, n=2–4, the bond length in NiFn, n=2–4, remains almost unchanged (Table S3.5). This different trend for nickel and its predecessors can be traced back to fundamental aspects of their atomic structures, such as a higher nickel ionization energy. This leads to a considerable covalent character of the Ni−F bonds, associated with a low-spin triplet electronic ground-state of NiF4 and a considerable radical character of the fluorine ligands (Figure S11).7, 8
From these experiments we conclude that NiF2, initially formed from the reaction of nickel atoms and elemental fluorine, can be regarded as chemically inert to elemental fluorine, but reacts rapidly with atomic fluorine radicals to form NiF3 and further to NiF4. It has also been shown that the reaction of Ni atoms with elemental F2 to produce NiF2 requires UV photolysis to yield appreciable quantities of product under the cryogenic conditions applied here. Given the highly exothermic reaction energy of this reaction (Table 2), this observation indicates a considerable reaction barrier and the necessity for fluorine radicals.
|
ΔE° (kJ mol−1), CCSD(T)/AVTZ-DK |
|---|---|
Ni + F → NiF |
−426.16 |
NiF + F → NiF2 |
−507.44 |
NiF + F2 → NiF3 |
−517.91 |
NiF2 + F → NiF3 |
−163.50 |
NiF3 + F → NiF4 |
−76.04 |
To summarize, molecular NiF4 (D2d) and NiF3 (D3h) were produced, isolated in solid noble-gas matrices, and spectroscopically identified aided by quantum-chemical calculations. Their weak Ni−F bonds and a considerable fluorine radical character make these molecular species very powerful fluorination and oxidation agents.
Acknowledgements
We gratefully acknowledge the Zentraleinrichtung für Datenverarbeitung (ZEDAT) of the FU Berlin for the allocation of computer time. We thank the DFG (HA 5639/10) for financial support. L.L. thanks the China Scholarship Council (CSC) for a PhD fellowship. AKS thanks the University of Hull for a PhD studentship. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 387284271—SFB 1349. S.R. thanks the late Dr. B. Schimmelpfennig for fruitful discussions. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




