Carbodicarbene Bismaalkene Cations: Unravelling the Complexities of Carbene versus Carbone in Heavy Pnictogen Chemistry
Abstract
We report a combined experimental and theoretical study on the first examples of carbodicarbene (CDC)-stabilized bismuth complexes, which feature low-coordinate cationic bismuth centers with C=Bi multiple-bond character. Monocations [(CDC)Bi(Ph)Cl][SbF6] (8) and [(CDC)BiBr2(THF)2][SbF6] (11), dications [(CDC)Bi(Ph)][SbF6]2 (9) and [(CDC)BiBr(THF)3][NTf2]2 (12), and trication [(CDC)2Bi][NTf2]3 (13) have been synthesized via sequential halide abstractions from (CDC)Bi(Ph)Cl2 (7) and (CDC)BiBr3 (10). Notably, the dications and trication exhibit C Bi double dative bonds and thus represent unprecedented bismaalkene cations. The synthesis of these species highlights a unique non-reductive route to C−Bi π-bonding character. The CDC-[Bi] complexes (7–13) were compared with related NHC-[Bi] complexes (1, 3–6) and show substantially different structural properties. Indeed, the CDC ligand has a remarkable influence on the overall stability of the resulting low-coordinate Bi complexes, suggesting that CDC is a superior ligand to NHC in heavy pnictogen chemistry.
Bi double dative bonds and thus represent unprecedented bismaalkene cations. The synthesis of these species highlights a unique non-reductive route to C−Bi π-bonding character. The CDC-[Bi] complexes (7–13) were compared with related NHC-[Bi] complexes (1, 3–6) and show substantially different structural properties. Indeed, the CDC ligand has a remarkable influence on the overall stability of the resulting low-coordinate Bi complexes, suggesting that CDC is a superior ligand to NHC in heavy pnictogen chemistry.
Introduction
The chemistry of bismuth has recently seen a surge in interest due in part to the stabilization of reactive bismuth complexes with novel electronic structure,1 and the utilization of low-valent and/or low-coordinate bismuth compounds in catalysis.2 Bismuth has also been the subject of green chemistry efforts as its non-toxic nature sets it apart from the lighter pnictogen elements (i.e., P, As, Sb).3
In low-coordinate bismuth chemistry, the discovery of homometallic Bi=Bi bonds4 and monometallic Bi complexes stabilized by N-donor ligands4l, 5 has dominated the chemical literature. In contrast, stabilizing reactive Bi centers using monodentate C-donors (e.g., carbenes) to access subvalent Bi complexes is rare.6 It is noteworthy that while the carbene chemistry of the lighter pnictogen elements is well-established and continues to lead to new avenues of research,7 carbene-bismuth compounds have presented significant experimental challenges and thus the field is severely underdeveloped.8 Indeed, Dutton and co-workers reported the first N-heterocyclic carbene (NHC)-bismuth complexes only six years ago,9 while those of phosphorus have been known for decades.9, 10 Recently, our laboratory has been exploring the coordination chemistry of bismuth with electronically diverse carbene ligands, which led to the synthesis of the first cyclic(alkyl)(amino) carbene (CAAC)-supported BiIII compounds.11 While the reaction of CAAC-Bi(Ph)Cl2 with traditional reducing agents (e.g., Na, K, KC8) resulted in rapid decomposition, utilization of the subvalent (CAAC)2Be0 complex afforded the first carbene-bismuthinidene, CAAC-BiIPh (Figure 1).6 The bonding interaction between the carbene carbon and the large Bi atom renders the two-coordinate carbene-bismuthinidene extremely reactive in solution, decomposing to Bi metal and free carbene. The thermal instability of the complex largely results from the destabilization of the 2pπ-6pπ interaction (i.e., weak donor-acceptor interaction and weak backdonation of the π-symmetric Bi lone pair to the carbene p-orbital) (Figure 1). As a result, it was of interest to investigate the effect of cationization and the reverse bonding situation, with both σ- and π-donation from carbon to bismuth. While a number of bismuth cations have been reported,12 the vast majority of such compounds feature anionic ancillary ligands. Recently, Mebs and Beckmann described the synthesis of a donor-free bismuth carbene analogue supported by bulky m-terphenyls (Figure 1, A).13 As demonstrated by Okuda, protonolysis of a tris(allyl)bismuth species results in the formation of a bis(allyl)bismuth monocation (Figure 1, B). Burford and co-workers reported a series of bis(bipyridine)pnictinium complexes. Interestingly, all of the pnictogen complexes were ionic with the exception of bismuth, which was described as a bis(bipyridine) adduct of bismuth trifluoromethanesulfonate (Figure 1, C).14
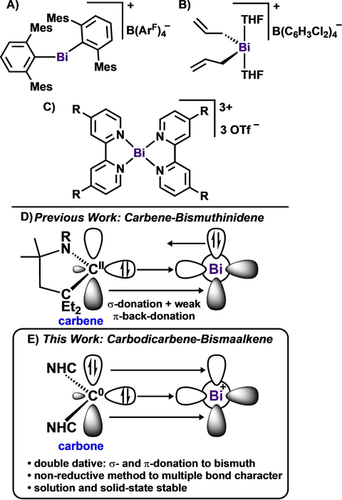
Representative examples of bismuth cations (A–C). Previous work: carbene-bismuthinidene (D). This work: carbodicarbene-bismaalkene cations.
Carbodicarbenes (CDC),15 a type of carbone,16 are ideal ligands for stabilizing low-coordinate electrophilic bismuth cations because of their strong nucleophilic character, which is superior to both NHCs and CAACs.17 Unlike carbenes, carbones can serve as two- or four-electron donors.18 While the first carbone was isolated in 1961 by Ramirez,19 CDCs were first theorized by us (G.F.)15a and their synthesis was achieved by Bertrand.15b Since the original report the library of available CDC ligands has been expanded,20 however, the utilization of this ligand framework in main-group chemistry remains scarce.21 Herein, we describe the first complexation reactions of CDC with bismuth, namely phenylbismuth dichloride, PhBiCl2, and bismuth tribromide, BiBr3. These complexes undergo halide abstraction reactions to give mono-, di-, and tri-positve bismuthenium ions. Notably, the CDC-bismuth cations represent a new carbon-bismuth bonding motif, with a double dative bond from carbon to bismuth. To the best of our knowledge, the C Bi interaction in compound 9 is the shortest known C−Bi+ bond, representing an unprecedented cationic bismaalkene. Moreover, the formation of the dications and trication demonstrate a non-reductive method to achieve heteronuclear C=Bi double bond character. Neutral pnictaalkenes C=E (E=pnictogen) are well-established for C=P22 and C=As23 multiple bonds. However, for the heavier elements, compounds containing C=Sb24 and C=Bi6, 24d, 25 multiple bonds are rare and synthetically challenging. For pnictaalkene monocations there are reports for [C=P]+ and [C=As]+,22d, 26 but to the best of our knowledge there are no structurally characterized examples pnictaalkene dications, [C=E]2+ (Figure 2). Interestingly, there is one example of a phosphaallene trication.21d In this manuscript, we have synthesized the first examples of [C=Bi]+ (8, 11), [C=Bi]2+ (9, 12), and [C=Bi=C]3+ (13).
Bi interaction in compound 9 is the shortest known C−Bi+ bond, representing an unprecedented cationic bismaalkene. Moreover, the formation of the dications and trication demonstrate a non-reductive method to achieve heteronuclear C=Bi double bond character. Neutral pnictaalkenes C=E (E=pnictogen) are well-established for C=P22 and C=As23 multiple bonds. However, for the heavier elements, compounds containing C=Sb24 and C=Bi6, 24d, 25 multiple bonds are rare and synthetically challenging. For pnictaalkene monocations there are reports for [C=P]+ and [C=As]+,22d, 26 but to the best of our knowledge there are no structurally characterized examples pnictaalkene dications, [C=E]2+ (Figure 2). Interestingly, there is one example of a phosphaallene trication.21d In this manuscript, we have synthesized the first examples of [C=Bi]+ (8, 11), [C=Bi]2+ (9, 12), and [C=Bi=C]3+ (13).
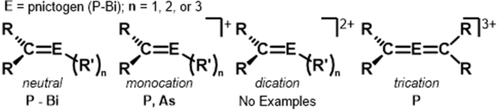
Structurally characterized examples of neutral and cationic pnictaalkenes.
In addition to the CDC-[Bi] complexes (7–13), we have prepared the first examples of carbene-supported di(organo)bismuthenium ions (3–5), which form through a THF-promoted rearrangement pathway, or by direct coordination of a sterically demanding carbene ligand. Experimental and theoretical analyses of NHC/CAAC- and CDC-bismuth cations clearly highlight the observed differences in stability and chemical bonding between carbene- and carbone-stabilized bismuth species.
Results and Discussion
Synthesis of NHC-supported Bismuthenium Complexes
We hypothesized that a strong monodentate neutral donor ligand would aid in the preparation of low-coordinate bismuth cations. Our group recently reported 1 (Scheme 1), which is supported by two NHC ligands (4,5-dimethyl-1,3-diisopropylimidazolin-2-ylidene).27 In an effort to synthesize an NHC supported monocation, we reacted [(NHC)BiPh2(μ-Cl)]2 with silver hexafluoroantimonate (AgSbF6). Instead of the expected halide abstraction, the NHC transferred from Bi to Ag to give compound 2. This represents an unusual case of reverse-transmetalation,28 where the carbene transfers to the silver-containing halide abstraction reagent. The formation of compound 2 was ascertained by single-crystal X-ray diffraction (Figure S40). Interestingly, recrystallization of [(NHC)BiPh2(μ-Cl)]2 from a THF/hexane mixture led to the formation of our target bismuthenium ion, [(NHC)BiPh2]+, which contains a dichlorodiphenylbismuthate counteranion, [Ph2BiCl2]− (3). Therefore, THF allows for the rearrangement of 1 to 3 by promoting NHC ligand dissociation and chloride abstraction to form the [Ph2BiCl2]− anion. The direct reaction of more sterically crowded carbene ligands, 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazole-2-ylidene (SIPr) and (2,6-diisopropylphenyl)-4,4-diethyl-2,2-dimethyl-pyrrolidin-5-ylidene (CAAC), with [Ph2Bi(μ-Cl)]2 led to cationic complexes (4–5) isostructural to that of compound 3. Indeed, no reaction occurs when [(SIPr)BiPh2][Ph2BiCl2] (4) or [(CAAC)BiPh2][Ph2BiCl2] (5) is reacted with an additional equivalent of carbene. The rearrangement chemistry and solvent-/carbene-promoted cationization observed is consistent with relatively weak carbene-Bi interactions that can be difficult to control. X-ray crystal structures for compounds 3–5 are shown in Figure S41.
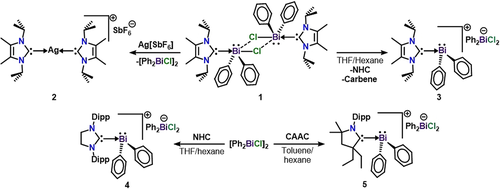
Synthesis of bismuth cations supported by N-heterocyclic carbenes and a cyclic(alkyl)(amino) carbene.
We hypothesized that the stronger electron donor properties of CDC compared to NHC would promote significant Bi−Cl bond elongation, allowing for facile halide abstraction. To draw comparisons between the two ligands and to test our hypothesis, we synthesized an NHC complex of phenylbismuth dichloride, [(NHC)Bi(Ph)Cl2]2, as well as the CDC analogue, (CDC)Bi(Ph)Cl2. NHC was allowed to react with (THF)Bi(Ph)Cl2 for 1 hour at room temperature in benzene (Scheme 2). After purification, compound 6 was obtained as a white solid in 75 % yield. The 1H NMR spectrum revealed a well-defined heptet at 4.95 ppm (J=7.0 Hz), representing the methine environment of a coordinated NHC ligand. Similarly, CDC was reacted with (THF)Bi(Ph)Cl2 in THF at room temperature, which forms a deep yellow precipitate upon addition. After filtration and drying under vacuum, (CDC)Bi(Ph)Cl2 (7) is obtained in 69 % yield. A broad peak at 5.22 ppm was observed in the 1H NMR spectrum for 7 in CD2Cl2, representing the methine proton of a new CDC coordination environment. In the 13C NMR, a sharp singlet at 189.3 ppm is representative of the carbone carbon atom, this is only slightly downfield of the carbene carbon in compound 6 (185.6 ppm).
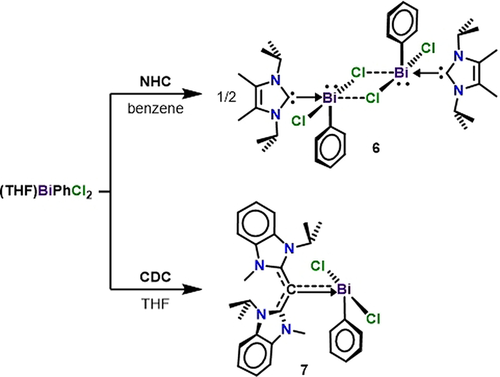
Synthesis of NHC- and CDC- supported phenylbismuth dichloride (NHC=4,5-dimethyl-1,3-diisopropylimidazolin-2-ylidene; CDC=bis(1-isopropyl-3-methyl-benzimidazol-2-ylidene)methane.
Colorless single-crystals of compound 6 were obtained from a saturated benzene solution at room temperature, while yellow crystals of compound 7 were obtained from a THF/hexane (1:2) layered mixture at room temperature (Figure 3). There are two notable differences between the solid-state structures of 6 and 7. (1) The NHC-supported bismuth complex 6 is dimeric and the CDC coordinated compound 7 is monomeric, suggesting that the strong electron donor properties of CDC negates the necessity for dimerization due to an electronically saturated bismuth atom. (2) The carboneC−Bi bond length in 7 [2.249(6) Å] is significantly shorter than the carbeneC−Bi bond length in 6 [2.346(2) Å]. This is a result of a partial π-donation from CDC to Bi in the former. As predicted, the average Bi−Cl bond lengths for 7 (2.734 Å) are longer than those in 6 (2.694 Å). Due to the observed differences in the metrical data we reasoned that CDC should lead to bismuth compounds with multiple bond character and enhanced stability. Thus, CDC-Bi cations were targeted as a platform to access bismaalkene-type species, which unlike the lighter pnictogen congers are extremely rare.
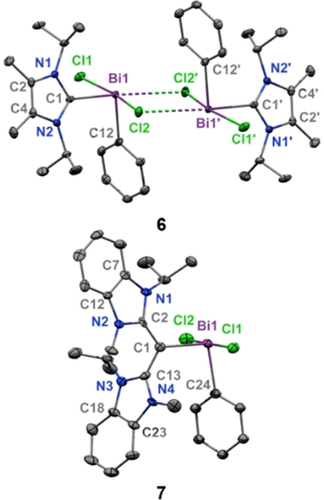
Molecular structures of 6 and 7 (thermal ellipsoids at 50 % probability; H atoms omitted for clarity). 6: C1-Bi1: 2.346(2); Bi1—Cl2′: 3.2473(7); Bi1-Cl1: 2.6857(6); Bi1-Cl2: 2.7031(6). Cl1-Bi1-Cl2: 166.081(18); Cl2-Bi1-C1: 99.21(8); Cl1-Bi1-C1: 84.96(5); C12-Bi1-Cl1: 92.21(6); C12-Bi1-Cl2: 91.65(6); C12-Bi1-C1: 99.21(8). 7: C1-Bi1: 2.249(6); Bi1-Cl1: 2.7693(14); Bi1-Cl2: 2.6989(15); Bi1-C24: 2.275(12); C1-C2: 1.393(8); C1-C13:1.445(8). Cl1-Bi1-Cl2: 176.21(5); Cl2-Bi1-C1: 90.52(15); Cl1-Bi1-C1: 93.23(15); C24-Bi1-Cl1: 89.8(5); C24-Bi1-Cl2: 89.2(5); C24-Bi1-C1: 97.2(6).
Synthesis of CDC-stabilized Mono-, Di-, and Tri-positive Bismuthenium Ions
In order to test our hypothesis that CDC would enable the formation of electropositive bismuth cations with increasing double bond character, we probed the reaction of 7 with one equivalent of AgSbF6 in dichloromethane (DCM) (Scheme 3). Orange needle-like X-ray quality crystals of the monocation (8) were obtained in 78 % yield from a THF/hexane (1:1) layered mixture at room temperature. The 1H NMR spectrum of 8 in [D8]THF revealed a broad resonance at 5.03 ppm for the methine proton of the coordinated CDC ligand which is slightly downfield compared to its starting material 7. Due to the more deshielding electrophilic bismuth center, the 13C NMR spectrum reveals a singlet downfield from that in 7 at 191.6 ppm attributed to the carbone carbon of 8. In pursuit of a dicationic species via a second halide abstraction, we added in an additional equivalent of AgSbF6 to 8 in DCM. Dark red X-ray quality crystals of the bismuthenium complex 9 were obtained in 43 % yield from a DCM/hexane (1:1) layered mixture at room temperature. A broad resonance attributed to the methine proton of coordinated CDC ligand in 9 is observed at 4.57 ppm in CD2Cl2, which is consistent with the trend observed for complexes 7 and 8. In this respect, the 13C NMR shows a peak at 209.7 ppm for the carbone carbon of 9, which is downfield from that of both 7 and 8, supporting substantial electron donation from CDC to bismuth.
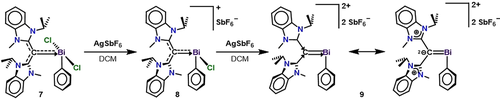
Synthesis of CDC-stabilized bismuth mono- and di-cations with increasing bismaalkene character.
The solid-state structures of cations 8 and 9 are shown in Figure 4. Both cations contain coordinating [SbF6]− counter anions, with Bi—F interaction distances of 2.904(2) Å (8) and 2.603(8) −2.740(7) Å (9). The CDC-bismuth bond length decreases in the order of 7 [2.249(6) Å] >8 [2.226(3) Å] >9 [2.157(11) Å], and these data support an increase in π-donor character from CDC as the bismuth center becomes increasingly more electrophilic. Notably, the C1−Bi1 bonds are significantly shorter than those in their NHC-bismuthenium congeners. Since carbones are π-basic rather than π-acidic, and the bismuth center is electrophilic, the C−Bi bond lengths are shortened due to substantial π-donation from the carboneC to Bi. The carboneC−Bi bond length in 9 is shorter than the CAACC−Bi bond in the subvalent carbene-bismuthindene [2.199(2) Å] and even rivals that of the bismuthio ylide (2.16 Å),25b and the aromatic C-Bi bonds of a bismabenzene (2.154–2.160(4) Å).25a Thus, 9 may be regarded a stabilized dicationic bismaalkene, which is unprecedented. It is noteworthy that while phosphaalkene species have been widely studied,22b, 22c, 29 structurally characterized examples of heavy bismuth analogues are virtually unexplored.
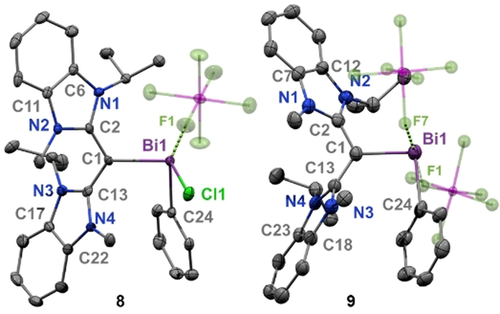
Molecular structures of 8 and 9 (thermal ellipsoids at 50 % probability; H atoms were omitted for clarity). 8: C1-Bi1: 2.226(3); Bi1-Cl1: 2.5573(9); C24-Bi1: 2.242(3); C1-C2: 1.417(5); C1-C13: 1.427(5); Bi1—F1: 2.904(2). C1-Bi1-Cl1: 102.82(9); C1-Bi1-C24: 93.60(13); C24-Bi1-Cl1: 93.86(10). 9: C1-Bi1: 2.157(11); Bi1-C24: 2.223(12); C1-C2: 1.444(15); C1-C13: 1.426(15); Bi1—F1: 2.603(8); Bi1—F7: 2.740(7); C1-Bi1-C24: 96.7(4).
In order to synthesize bismuthenium ions without weakly coordinating anion contacts, we reacted CDC with bismuth tribromide in THF overnight (Scheme 4). After workup, compound 10 was obtained as a red solid in 86 % yield. The 1H NMR spectrum of 10 shows a well-defined heptet (J=6.7 Hz) at 5.01 ppm representative of a sterically unrestricted CDC coordination environment; this is in contrast to the broad signals observed for 7, 8, and 9. Red single crystals of 10 suitable for X-ray diffraction analysis were obtained from a THF/hexane (2:1) layered mixture at room temperature.
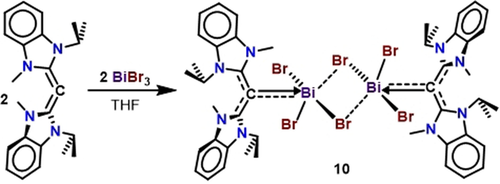
Synthesis of a bis-CDC-supported tribromobismuth dimer.
The solid-state structure of compound 10 shows a dimeric [(CDC)BiBr3]2 complex with bridging bromides (Figure 5). Each bismuth atom has adopted a distorted square pyramidal geometry when considering the bromide contacts. The CDC-bismuth bond [C1−Bi1: 2.292(9) Å] is shorter than that in compound 7 [2.249(6) Å]. The bismuth-bromide bond in the apical position of 10, [Bi1−Br2: 2.6629(16) Å], is shorter than the sum of ionic radii for Bi and Br (2.68 Å).30 The other two bismuth-bromide bonds are substantially longer [Bi1−Br1: 2.9196(12) Å and Bi1−Br3: 2.8390(12) Å], which support weaker Bi-Br interactions.
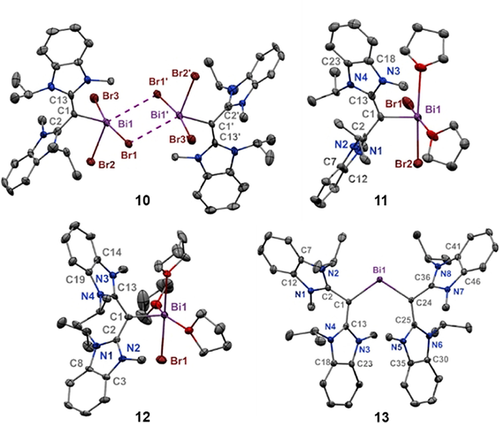
Molecular structures of 10–13 (thermal ellipsoids at 50 % probability; H atoms, counteranions and non-coordinating solvent omitted for clarity). 10: Bi1-C1: 2.292(9); Bi1-Br1: 2.9196(12); Bi1-Br2: 2.6483(11); Bi1-Br3: 2.8390(12); Bi1—Br1′: 3.4829(15); C1-C13: 1.367(13); C1-C2: 1.438(12). 11: Bi1-C1: 2.226(12); Bi1-Br2: 2.6629(16); Bi1-Br1: 2.7052(18); C1-C13: 1.395(17); C1-C2: 1.447(15). 12: Bi1-Br1: 2.6439(6); Bi1-C1: 2.199(5); C1-C2: 1.441(6); C1-C13: 1.433(6). 13: Bi1-C24: 2.166(2); Bi1-C1: 2.197(2); C1-C13: 1.424(3); C1-C2: 1.443(3); C24-C25: 1.436(3); C24-C36: 1.444(3). C24-Bi1-C1: 111.23(9).
While halide abstraction from compound 7 will only yield mono- and di-positive bismuthenium ions (8 and 9), compound 10 is a suitable starting material for synthesizing a tri-positive bismuthenium ion. Our initial synthetic approach involved the use of THF, which would provide additional support to the bismuth center by readily filling the coordination sphere upon halide abstraction. Interestingly, when compound 10 is reacted with AgSbF6, THF solvent polymerized into a tough insoluble solid material uncharacterizable by traditional spectroscopic methods. In an effort to circumvent this polymerization process, but still allow for THF coordination, we performed the reaction of 10 with two equivalents of AgSbF6 in DCM and added an aliquot of THF in situ (Scheme 5). Thus, the monocation 11 was isolated in 67 % yield. Unlike the monocation 8, compound 11 does not contain any anion interactions due to the coordination of THF solvent molecules to Bi. Attempts to form the dicationic product from the reaction of 10 with four equivalents of AgSbF6 and a variety of other halide abstraction reagents proved to be unsuccessful. This is in accordance with the observed difference in Bi−Br bond distances, which suggest that one Br atom is more tightly bound to Bi. However, facile halide abstraction was observed to generate the dication 12 when four equivalents of silver (bis-trifluoromethylsulfonyl)imide (AgNTf2) was reacted with 10. Similar to 11, compound 12 does not contain any anion contacts to the bismuth center. The 1H NMR integrations suggested that a third equivalent of THF coordinated to bismuth with respect to the monocation 11, to give a 1:3 THF to CDC ratio. Several attempts were made to synthesize a trication using this THF addition method, but all proved to be unsuccessful. We therefore hypothesized that the coordination of a second CDC ligand would render the last Bi−Br bond more labile due to the strong donor effect, thus weakening the Bi−Br bond. As such, the reaction 10 and two equivalents of CDC with six equivalents of AgNTf2 successfully gave the bis(CDC)bismuthenium trication 13 in 73 % yield. The shift pattern in the 1H NMR spectrum of 13 closely resembles that of a bis(CDC) dicationic(hydrido)boron complex,21f further supporting that two CDC ligands have coordinated to the Bi center.
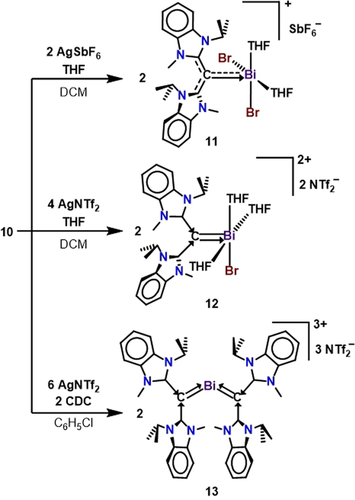
Synthesis of CDC mono-, di-, and tri-positive bismuthenium ions. Note: similar to compound 9, compounds 12 and 13 can be represented as resonance structures with their respective zwitterions as shown in Scheme 3.
Red needle-like crystals of 11 suitable for X-ray diffraction were obtained from a THF/hexane (1:1) layered mixture at room temperature. The crystal structure reveals a monocationic [(CDC)BiBr2(THF)2]+ core, and the bismuth center has adopted a square pyramidal geometry. The carboneC−bismuth bond of 11 [C1-Bi1: 2.226(12) Å] is slightly longer than that of 8, which is expected for a higher coordination number. Red plate-like crystals of compound 12 were obtained from a DCM/ether (2:1) mixture at room temperature. Similar to 11, the structure reveals a square pyramidal dicationic [(CDC)BiBr(THF)3]2+ core, with an additional THF occupying a coordination site. As a result of three coordinating THF molecules, the carboneC−Bi bond of 12 [C1−Bi1: 2.199(5) Å] is longer than 9 [C1−Bi1: 2.157(11) Å]. Dark blue plate-like crystals of compound 13 were obtained from a chlorobenzene/hexanes (10:1) mixture. The crystal structure of 13 revealed two CDC ligands coordinated to a BiIII center, with three [NTf2]− counter anions. The carboneC−bismuth bonds [Bi1−C24: 2.166(2) and Bi1−C1: 2.197(2) Å] are only slightly shorter than that in 12. The average allenic bond length has increased from 1.335 Å in the free ligand to 1.437 Å suggesting substantial electron donation from the carbone carbon to BiIII, and less back donation to the NHC moiety.
Bonding Analysis of the Bismuth and Bismuthenium Complexes
CDC-stabilized bismuthenium complexes 7–13 were calculated with quantum chemical methods using density functional theory (DFT) at the BP86-D3(BJ)/def2-TZVPP level.31 The nature of the chemical bonds between the CDC ligand and the remaining bismuthenium fragments [Bi] was investigated with energy decomposition analysis (EDA)32 in conjunction with natural orbitals for chemical valence (NOCV).33 The EDA-NOCV method32, 33 has been proven to give deep insights into the nature of the chemical bond in a variety of main-group compounds and transition metal complexes.34
The calculated equilibrium structures of 7–13 are shown in Figure S42 of Supporting Information. The calculated and experimental geometries are in agreement except for some Bi—F distances for the weakly coordinating anions in 8 and 9, which are considerably shorter than the experimental distances. This is also observed when the M06-2X functional is used for the geometry optimization.35 These differences are most likely due to intermolecular forces in the solid state. Therefore, we carried out the bonding analysis with the EDA-NOCV method using the calculated and the experimental geometries (Table S3–S5). Figure S42 displays the computed bond dissociation free energy (ΔG298) of the CDC−Bi bond at 298 K which ranges from 27.2–62.3 kcal mol−1, which indicates that the molecules are thermodynamically quite stable. We optimized the geometry of the compound 13 (CDC)2Bi[NTf2]3 and the free trication [(CDC)2Bi]3+. The agreement of the theoretical values of (CDC)2Bi[NTf2]3 with the experimental data is reasonably good, the deviation between the calculated and X-ray values is likely to be caused by solid-state forces. The calculated geometry of the free trication [(CDC)2Bi]3+ is only slightly different from the geometry in the presence of the counter ions in (CDC)2Bi[NTf2]3. The bond strength of the CDC ligands to bismuth in (CDC)2Bi[NTf2]3 has been estimated by calculating the bond dissociation energy for the reaction (CDC)2Bi[NTf2]3 → 2 CDC + Bi[NTf2]3. The computed value of D0 (ΔG298)=128.6 (88.2) kcal mol−1 suggests that the bonds are quite strong.
Table S5 shows the numerical EDA-NOCV results of 7–13 using the calculated geometries (experimental geometries are shown in Figure S42). The calculated intrinsic interaction energy (ΔEint) values are similar except for structures 8, 9 and 12, where the energy values using the experimental geometries are somewhat larger. However, the percentage contributions of the four components to the CDC-[Bi] interaction, i.e., Pauli repulsion ΔEPauli, dispersion forces ΔEdisp, electrostatic attraction ΔEelstat and orbital (covalent) interaction ΔEorb are very similar. This indicates that the bonding analysis using the calculated structures may safely be used for the experimentally observed species. The breakdown of the ΔEorb term into pairwise interactions ΔEorb(1)−ΔEorb(3) shows that the major contribution ΔEorb(1) comes from the σ donation of the CDC ligand [CDC]→[Bi]. The donation ΔEorb(2) of the π lone pair of the CDC ligand14 [CDC]→[Bi] is significantly weaker (Table S5). It becomes stronger when the experimental geometries are used, because the measured carbon-bismuth distances are shorter than the calculated values (Figure S42). The largest contribution of the [CDC]→[Bi] π donation is found for 9 where it amounts to 19 % of the total orbital interactions when the experimental geometry is used (Table S3). The third pairwise orbital contribution ΔEorb(3) is due to weak [CDC]←[Bi] σ backdonation. This is the complementary part of the overall σ bond. For the trication 13, the lowest values were provided by using the triplet states of [(CDC)2]2+ and Bi+ with the electron configuration (6s26py16pz1) for the atomic ion. The numerical results of the EDA-NOCV calculations are given in Table S5, which show also the xyz directions of the p AOs. The calculated results using other fragments are given in Table S7 of Supporting Information. We also calculated the other systems 7–12 using triplet fragments. The EDA-NOCV results in Table S8 show that the ΔEorb values are always much higher than provided by the singlet fragments shown in Table S5.
The results of the bonding analysis suggest that the NHCPh substituents of the CDC ligand serve as a sink for the electronic charge, because they are rotated out of the molecular frame so that the vacant π orbitals of NHCPh can interact with the occupied orbitals of the [Bi] donor. The related molecular orbitals representing double dative interaction is provided in Figure S43. Note that in moving from 7→8→9 or 10→11→12, with the increase in electrophilic character of Bi, both the absolute values of [CDC]→[Bi] σ and π donation gradually increases (from 10→11 the σ donation remains essentially the same), which agrees with the increasingly shorter carboneC−Bi bond distances.
The pairwise orbital interactions ΔEorb(1)−ΔEorb(3) can be assigned to specific donations with the help of the associated deformation densities Δρ. Figure S45 shows the shape of Δρ(1)−Δρ(3) of the pairwise interactions ΔEorb(1)−ΔEorb(3) in 9 (for the remaining systems, see Figure S45). The color of the charge flow is red→blue. It becomes obvious that, besides the σ donation and π backdonation, there is also polarization within the fragments.
We have further compared the bonding interactions in 7–13 with that in carbene-stabilized bismuthenium 3–5 and bismuth CAAC-Bi(Ph)Cl2 complexes.7 The optimized geometries of the four carbene adducts are shown in Figure S46. The numerical results of the EDA-NOCV calculations are given in Table S6. A comparison of the data in Tables S5 and S6 shows that the intrinsic interaction in the CDC complexes is larger than that in the carbene adducts. The most important information comes from the breakdown of ΔEorb into the pairwise orbital interactions between the ligands and the metal fragments. There is only one dominating σ donation ΔEorb(1) in the carbene complexes [carbene]→[Bi] besides the π backdonation [carbene]←[Bi] ΔEorb(2) (For associated deformation densities see Figure S47). In contrast, the CDC complexes exhibit two notable donor components from the ligand to the metal fragment. The remaining orbital interactions ΔErest consist of a large number of weak polarization terms within the fragments. Thus, the EDA-NOCV calculations signal a clear difference in the donor-acceptor interactions between the carbone and carbene ligands. This is best exemplified by comparing the results of the carbone complex CDC-Bi(Ph)Cl2 (7) with the analogous carbene adduct CAAC-Bi(Ph)Cl2. The total interaction energy ΔEint of 7 is only slightly higher (−81.5 kcal mol−1) than that of the carbene complex (−78.4 kcal mol−1) but the orbital interactions ΔEorb of the former carbone complex are much larger (−108.0 kcal mol−1) than for the latter carbene species (−98.0 kcal mol−1), which is mainly due to the additional contribution of the π bond (−7.3 kcal mol−1). The further strengthening of the orbital interactions is partly compensated by weaker electrostatic attraction in the carbone complex. It requires the analysis of the electronic structure in order to find that 7 and the other carbone complexes possess a double dative C Bi bond, which consists of a strong σ and a significantly weaker π component, whereas the carbene complexes exhibit only a σ bond C→Bi.
Bi bond, which consists of a strong σ and a significantly weaker π component, whereas the carbene complexes exhibit only a σ bond C→Bi.
Conclusion
In this study a series of carbene- and carbone-stabilized bismuthenium ions are reported. The CDC-Bi(Ph)Cl2 and [CDC-BiBr3]2 complexes were used as starting materials to afford structurally diverse bismuth monocations, dications, and trication. The dication 9 and trication 13 possess short Bi=C bonds due to the low-coordinate, double dative-type interaction to Bi. Thus, compounds 9 and 13 may be viewed as bismaalkene cations, which are hitherto unprecedented. The enhanced stability of the CDC-[Bi] complexes, compared to NHC-[Bi] species, clearly highlights the superior donor ability of CDC, and the importance of single atom σ- and π-donation in stabilization strategies employing neutral carbon-donor ligands. These interactions are also studied in detail with charge and energy decomposition analyses, which provide clear evidence that the carbone complexes CDC-[Bi] possess a double donor bond C Bi which consists of a strong σ and a significantly weaker π component, whereas the carbene complexes exhibit only a σ bond C→Bi. Due to the highly electrophilic nature of the CDC-bismuth cations, they are expected to possess interesting reactivity toward energy-relevant small molecules (CO, CO2, H2, etc.) and these studies are currently under investigation in our laboratory.
Bi which consists of a strong σ and a significantly weaker π component, whereas the carbene complexes exhibit only a σ bond C→Bi. Due to the highly electrophilic nature of the CDC-bismuth cations, they are expected to possess interesting reactivity toward energy-relevant small molecules (CO, CO2, H2, etc.) and these studies are currently under investigation in our laboratory.
Acknowledgements
We are grateful to the University of Virginia (UVA) for support of this work. G.F. and S.P. thank the German Research Foundation (DFG) for funding. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




