B−B Bond Nucleophilicity in a Tetraaryl μ-Hydridodiborane(4) Anion
Abstract
The tetraaryl μ-hydridodiborane(4) anion [2H]− possesses nucleophilic B−B and B−H bonds. Treatment of K[2H] with the electrophilic 9-H-9-borafluorene (HBFlu) furnishes the B3 cluster K[3], with a triangular boron core linked through two BHB two-electron, three-center bonds and one electron-precise B−B bond, reminiscent of the prominent [B3H8]− anion. Upon heating or prolonged stirring at room temperature, K[3] rearranges to a slightly more stable isomer K[3 a]. The reaction of M[2H] (M+=Li+, K+) with MeI or Me3SiCl leads to equimolar amounts of 9-R-9-borafluorene and HBFlu (R=Me or Me3Si). Thus, [2H]− behaves as a masked [:BFlu]− nucleophile. The HBFlu by-product was used in situ to establish a tandem substitution-hydroboration reaction: a 1:1 mixture of M[2H] and allyl bromide gave the 1,3-propylene-linked ditopic 9-borafluorene 5 as sole product. M[2H] also participates in unprecedented [4+1] cycloadditions with dienes to furnish dialkyl diaryl spiroborates, M[R2BFlu].
The “Umpolung” strategy, by which the donor or acceptor properties of a reaction center are interchanged,1 has revolutionized organic synthesis. As prominent examples, organolithium or Grignard reagents react in an inverse manner compared to the innate electrophilic nature of their organohalide precursors and, thereby, substantially increase the options for synthetic planning. A similar breakthrough could be anticipated for the ever-growing area of boron chemistry, if nucleophilic boron species were readily available. Organoboranes, in particular, are vital compounds with applications ranging from advanced synthesis to catalysis and materials science.2 However, with six valence electrons and a vacant pz orbital, trivalent boron centers are naturally electrophiles that require nucleophilic partners for polarity-driven transformations. Polarity inversion of the electropositive semimetal boron is even harder to achieve than in the case of carbon. Thus, despite considerable efforts, only a handful of isolable and well-characterized nucleophilic boron compounds have been reported, among them the 1,3,2-diazaborole-derived boryllithium of Nozaki, Yamashita and co-workers,3 the magnesium boryl complexes of Hill and co-workers,4 the carbene-stabilized tetraphenylborolyl anion of Braunschweig et al.,5 the hydride-stabilized dibenzoborole anion of Wagner and co-workers,6 and the tricyanoborate dianion of Willner, Finze, and co-workers.7
In terms of real-world applications, the currently most practical approach for generating nucleophilic boron species is based on the treatment of tetra(organyloxy)diboranes(4) with Lewis bases, mainly alkoxides [OR′]−.8 The resulting monoadducts [(RO)2B−B(OR)2(OR′)]− are polarized in such a way that their B(sp2)−B(sp3) bonds can undergo heterolytic cleavage with liberation of B(OR)2(OR′) and transfer of [:B(OR)2]− anion equivalents to suitable substrates.9 Can this concept be generalized to other diboranes(4) which may act as surrogates for a broader variety of [:BR2]− anions, especially those without π-donating heteroatom substituents?
Our group has recently gathered evidence for the transient occurrence of [FluB−BFlu(R)]− (HBFlu=9-H-9-borafluorene) intermediates, which are capable of releasing a 9-borafluorenyl anion [:BFlu]− that subsequently undergoes either carbene-type C−H bond activation or nucleophilic substitution reactions (A; Figure 1).10, 11 If we consider [FluB−BFlu(R)]− as containing a [:BFlu]− moiety stabilized via formation of an adduct through its lone pair of electrons with a Lewis acidic 9-R-9-borafluorene molecule, it would be justified to regard the entire electron density between the two boron atoms as the actual nucleophilic entity (cf. the related idea of nucleophilic B−H bonds12). Other species possessing nucleophilic B−B σ bonds include the three-membered ring species B,13 the doubly guanidinate-bridged diborane(4) C,14, 15 and compound D with a B−B-supporting BHB two-electron, three-center bond (Figure 1).16
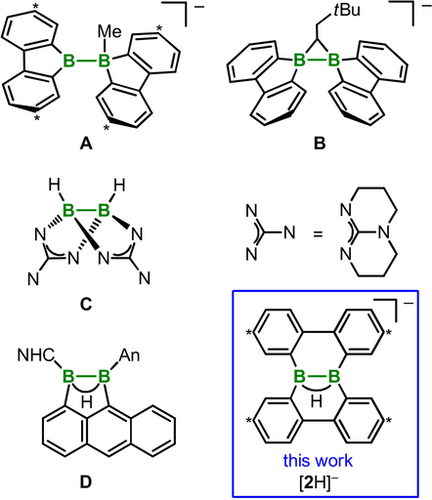
Known ditopic boron compounds A–D with electron-rich B−B cores (NHC=N-heterocyclic carbene, An=9-anthryl) and the nucleophilic anion [2H]− investigated in this work. Carbon atoms marked with asterisks bear tBu substituents.
Among other reactions, B and C undergo clean protonation of their B−B bonds. D coordinates as a neutral σB-B ligand to CuI complexes and was advertised as a “bottleable neutral analogue” of the pristine [B2H5]− anion, which, although not existing in free form, has been stabilized in the coordination sphere of various transition-metal complexes.17, 18
Quantum-chemical calculations predict a non-classical, hydrogen-bridged minimum structure for [B2H5]−; a classical isomer [H2B−BH3]− is only 7.8 kcal mol−1 higher in energy (first order saddle point).18 The simple shift of one hydrogen atom from the bridging to a terminal position dramatically influences the nodal structures of the respective highest occupied molecular orbitals (HOMOs; Figure 2 a): 1) Nonclassical [B2H5]− features lobes of π symmetry at its B−B bond with a proton immersing into the π orbital and has fittingly been described as a complex between [:BH2]− and BH3 (Figure 2 b; R=H).19 2) Classical [B2H5]− is characterized by a B−B σ bond and, therefore, related to the [:B(OR)2]− source [(RO)2B−B(OR)2(OR′)]−.20, 21 We thus wondered whether isolable organic derivatives [B2R4H]− of [B2H5]− could behave as surrogates of [:BR2]− anions (Figure 2 b; cf. also the corresponding reactivity of A).
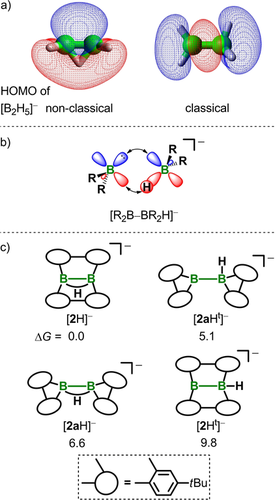
a) Plots of the highest occupied molecular orbitals (HOMOs) of non-classical (minimum) and classical (saddle point) [B2H5]−. b) Systems [R2B−BR2H]− can been described as complexes between [:BR2]− and BR2H (R=H, organyl).19 c) Schematic molecular structures and relative Gibbs free energies [kcal mol−1] of four isomers of [2H]−, all of them representing minima on the potential energy surface.21
As a promising candidate we identified the recently reported compound [2H]− (see Figure 1).22, 23 A computation of four conceivable isomers of [2H]− revealed all to be minima on the potential energy surface (Figure 2 c).21 The μ-hydridodiborane(4) anion [2H]− represents the lowest energy structure, but the rearranged classical bis(9-borafluorenyl) isomer [2 aHt]− containing a ligand-unsupported B−B bond and a terminal hydrogen substituent also possesses a favorable geometry (ΔG=+5.1 kcal mol−1 relative to [2H]−; see the Supporting Information for more details). The Wagner–Meerwein-type 1,2-phenyl shifts underlying the rearrangement [2H]−→[2 aHt]− have repeatedly been encountered in transformations of [2H]−-type systems.2, 6, 10, 22-30
Herein, we disclose highly selective substitution and electrocyclic reactions starting from [2H]− and uncharged electrophiles or carbonium/silylium ion transfer reagents. Key features of our system's reactivity will be explained by taking into account both the non-classical minimum structure [2H]− and the classical isomer [2 aHt]−, the HOMOs of which closely resemble those of their [B2H5]− congeners (see the Supporting Information).
The potassium salt K[2H] was first prepared through the reduction of the diborane(6) 2H2 with KC8 (58 % yield);22 essentially quantitative yields of M[2H] (M+=Li+, K+) were later obtained by deprotonation of 2H2 with the bulky Brønsted bases (Me3Si)3CLi or (Me3Si)2NK (Scheme 1).23, 31 Compound 2H2, in turn, forms upon thermal rearrangement of the 9-H-9-borafluorene dimer (1)2.25, 27
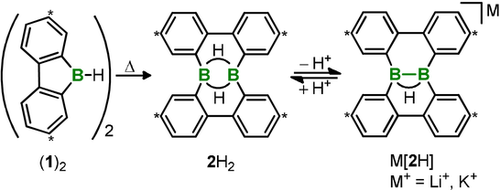
Synthesis of M[2H] (M+=Li+, K+) through thermal rearrangement of the dimeric 9-H-9-borafluorene (1)2 and deprotonation of the intermediate 2H2.23 Carbon atoms marked with asterisks bear tBu substituents.
Previous mechanistic studies on B2pin2-activation reactions with strong nucleophiles MR/MR2 or MOR have shown a possible influence of the cations M+/M2+.8 Our reactivity studies on [2H]− were thus carried out using both its Li+ and K+ salts. Except for a few cases, which are explicitly mentioned herein, no significant differences were observed. We will, therefore, restrict the discussion of obtained results to the cases of those salts that provided the most comprehensive set of information. In a proof-of-principle experiment to demonstrate the Lewis basic behavior of [2H]−, we had already protonated its B−B bond and thereby regenerated 2H2.23 Are other main-group Lewis acids also sufficiently electrophilic to form stable adducts with [2H]−?
To answer this question, we treated K[2H] in THF with 1 equiv of the 9-H-9-borafluorene adduct 1⋅THF22 and confirmed a quantitative and selective conversion by NMR spectroscopy. Crystals of the product were grown directly from the reaction mixture through slow evaporation of the solvent at room temperature. According to an X-ray diffraction analysis, the B3 cluster K[3] had formed (Scheme 2 a), which can be viewed as a hexaaryl derivative of the famous octahydrotriborate [B3H8]−.32
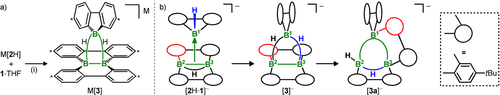
a) Synthesis of the B3 cluster M[3] through the addition of 9-H-9-borafluorene (1) to M[2H] (M+=Li+, K+). b) Postulated reaction pathway leading from an unobserved primary Lewis pair [2H⋅1]− to the isolable kinetic and thermodynamic products [3]− and [3 a]−, respectively. (i) [D8]THF, room temperature. Carbon atoms marked with asterisks bear tBu substituents.
The cluster anion [3]− consists of the intact subunits contributed by the two starting materials (Figure 3 a):33 a planar 9-borafluorene moiety is oriented roughly orthogonal to the B2−B3 bond within the twisted, doubly boron-doped dibenzo[g,p]chrysene system [the torsion angle between the C−C bonds connecting the phenyl rings Ph(C21)/Ph(C31) and Ph(C41)/Ph(C51) amounts to 40.9(3)°]. Two H atoms residing at bridging positions between B1/B2 and B1/B3 have been located in the difference Fourier map. The B−B distances in these BHB two-electron, three-center bonds are close to the length of the electron-precise B2−B3 bond [B1−B2=1.797(6) Å, B1−B3=1.833(6) Å versus B2−B3=1.833(5) Å].
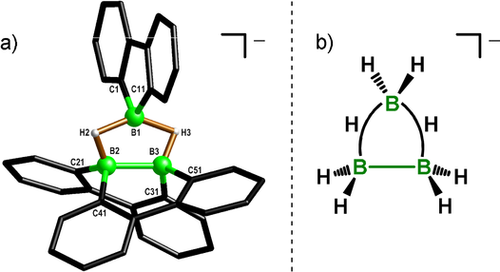
a) Molecular structure of the B3 cluster [K(thf)2][3] in the solid state.33 The [K(thf)2]+ cation, tBu groups, and all carbon-bonded H atoms have been omitted for clarity. B−B distances [Å] in [K(thf)2][3]: B1−B2=1.797(6), B1−B3=1.833(6), B2−B3=1.833(5). b) Lewis structure of the parent [B3H8]− anion of [3]−.
An 1H NMR spectrum recorded on a [D8]THF solution of the single crystals revealed two signal sets (integral ratio=2:4), each of them characteristic of one 1,2,4-substituted benzene ring. The same was true for the 13C{1H} NMR spectrum. The twisted dibenzo[g,p]chrysene fragment of K[3] thus undergoes rapid inversion movements such that Ph(C1)/Ph(C11) on the one hand and Ph(C21)/Ph(C31)/Ph(C41)/Ph(C51) on the other become magnetically equivalent on the NMR time scale. All the C−H resonances of K[3] are upfield-shifted compared to those of the starting materials. This effect is particularly pronounced for the H nuclei ortho to the borafluorene boron atom [Δδ(o-H)=1.43 ppm], which directly point into the magnetic anisotropy shielding cones of facing aryl rings (Figure 3 a). One resonance at δ=3.32 ppm (2 H) is assignable to both BHB atoms; the 11B NMR spectrum of K[3] exhibits two signals at δ=4.8 and −17.6 ppm.
When samples of K[3] in [D8]THF were heated at 55 °C for several hours, new NMR resonances developed, in line with the formation of a less-symmetric compound. The final 1H, 11B, and 13C{1H} NMR spectra obtained were all virtually identical to those of the known, partially ring-opened B3 cluster Li[3 a].34 Furthermore, the identity of the rearrangement product as K[3 a] was confirmed by X-ray crystallography (see Scheme 2 b for a schematic representation and Figure S38 for a plot of the solid-state structure of [K(thf)2][3 a]). The rearrangement K[3]→K[3 a] also occurs at room temperature; however, a period of >5 days is required until the first signs of K[3 a] are detectable by NMR spectroscopic analysis of dilute solutions of K[3] (higher concentrations increase the rearrangement rate).
The reaction sequence leading to K[3 a] likely starts with σ donation from the B−B bond of [2H]− into the vacant boron pz orbital of 1 to afford [2H⋅1]− (Scheme 2 b). Even though we have never observed this initial Lewis acid-base adduct by NMR spectroscopy, DFT calculations predict it to be a minimum on the potential energy surface.21 However, the actually isolated primary cluster K[3] and the rearranged K[3 a] are lower in energy by 8.9 and 9.2 kcal mol−1, respectively (the calculated energy difference of 0.3 kcal mol−1 between K[3] and K[3 a] appears to be underestimated in light of the empirically observed reactivities of both isomers, likely because of the omission of the counter cations and/or tBu groups in the calculations). On the way from K[3] to K[3 a], the μ-H atoms marked in black and blue have to shift to a terminal position at B2 and a bridging position between B2 and B3, respectively (Scheme 2 b). The red-colored aryl ring, in turn, moves from B2 to B1. The fast and quantitative formation of M[3] in THF demonstrates that M[2H] is sufficiently nucleophilic to outcompete even excess THF as a ligand of 1. B3 clusters comparable to [3]− have been obtained by Himmel and co-workers from the reaction of C (Figure 1) with sources of borinium cations [BR2]+ (R=H, alkyl).35-37
In a first endeavor to also exploit the “Umpolung” reactivity of Li[2H] for establishing B−C bonds, we treated a solution of the compound in [D8]THF with excess neat MeI. 1H NMR spectroscopic analysis of the reaction progress revealed the immediate consumption of Li[2H] and the presence of three components in the intermediate mixture: residual MeI, the target molecule 9-Me-9-borafluorene (4),10 and Li[3] (stoichiometric ratio, Li[3]:4=1:1). Li[3] slowly reacted further and after 3 days, the sole products contained in the sample were 4 and 1⋅THF in equimolar amounts (Scheme 3). Clearly, the nucleophilic attack of the B−B bond on MeI is fast and generates 1 equiv of 1⋅THF as a by-product of the substitution product 4. 1⋅THF initially traps the Lewis base [2H]− in the form of Li[3], but in the long run gives way to its competitor MeI because the cluster formation is reversible (note that K[3 a] is inert toward MeI).
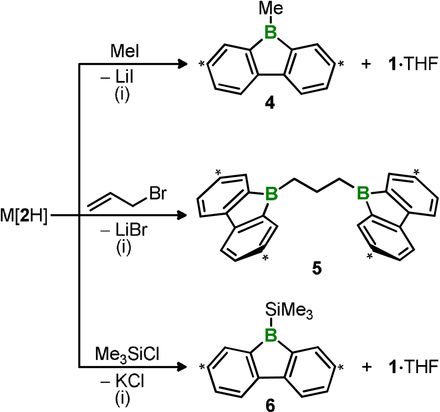
Formal cleavage of M[2H] (M+=Li+ or K+) into M[:BFlu] and 1⋅THF upon treatment with MeI, C3H5Br, or Me3SiCl and the resulting formation of B−C/Si bonds (4–6) through nucleophilic substitution. In the case of 5, the by-product 1⋅THF was used as an in situ hydroboration reagent to prepare the ditopic borane. (i) [D8]THF, room temperature. Carbon atoms marked with asterisks bear tBu substituents.
There are two variants of the plausible mechanistic pathway leading from Li[2H] and MeI to 4 and 1⋅THF: a) Similar to [pinB-Bpin(OR′)]−, which liberates 1 equiv of Bpin(OR′) upon transfer of a [:Bpin]− anion, the thermally accessible B(sp2)−B(sp3) isomer [2 aHt]− would release 1⋅THF when it generates the nucleophilic [:BFlu]− (Figure 4 a). b) MeI interacts with the B−B bond of [2H]− to furnish an intermediate of type 2H2, in which one B⋅⋅⋅B-bridging H+ ion is replaced by a [CH3]+ cation. Two subsequent 1,2-phenyl shifts would then generate the observed products 4 and 1⋅THF (Figure 4 b). Notably, the by-product 1⋅THF can still be employed as a valuable reagent in tandem substitution-hydroboration reactions to afford ditopic boron-containing Lewis acids (cf. the conceptually related use of Bpin(OR′) in the alkoxide-catalyzed diboration8 of alkenes with B2pin2). To confirm this claim, we performed the transformation of Li[2H] with allyl bromide and quantitatively obtained the known 1,3-bis(9-borafluorenyl)propane 510 (Scheme 3).
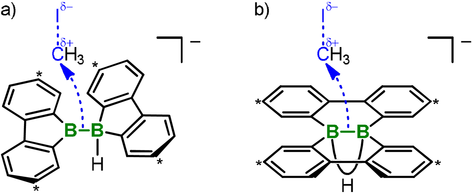
Nucleophilic attack of the B−B bonds of [2 aHt]− (a) or [2H]− (b) on the carbon electrophile MeI. Carbon atoms marked with asterisks bear tBu substituents.
Our “Umpolung” approach to the generation of boron–element bonds is not restricted to element=carbon, but can be extended to the heavier homologue silicon: in a similar scenario as described above for MeI, Me3SiCl converts K[2H] into a 1:1 mixture of the silyl borane 6 and 1⋅THF (Scheme 3). Full conversion was only achieved with the K+ salt, whereas the transformation of Li[2H] stopped at the stage where 6 and the B3 cluster Li[3] had formed in equal amounts. The driving force provided by the precipitation of KCl from THF is clearly required to push the reaction to completion.
The proposed key intermediate [:BFlu]− is not only a nucleophilic boryl anion, but also an isoelectronic analogue of ambiphilic diaryl carbenes, :CAr2. Cycloadditions are signature reactions of carbenes. With this in mind, we next studied the behavior of K[2H] toward excess 2,3-dimethyl-1,3-butadiene (DMB) and observed a clean conversion into the spiroborate K[7] and the double hydroboration product 8 in a molar ratio of 2:1 (Scheme 4; see the Supporting Information for the crystallographically determined molecular structure of K[7]). We explain the absence of a DMB-monohydroboration product by assuming that its remaining nonconjugated C=C bond is more reactive toward 1⋅THF than the resonance-stabilized π system of the starting diene.38 The atom connectivity in 8 has also been confirmed by means of an X-ray crystal-structure determination.39 The effective trapping of [:BFlu]− in the form of K[7] provides additional proof for the transient appearance of this proposed intermediate. It also illustrates the first [4+1] cycloaddition reaction of a boryl anion, a potentially very useful access route to the emerging materials class of organic spiroborates.40
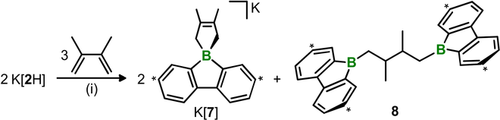
The spiroborate K[7] and its by-product 8, resulting from [4+1] cycloaddition and double hydroboration reactions, respectively, occur upon mixing of K[2H] and DMB. (i) [D8]THF, room temperature. Carbon atoms marked with asterisks bear tBu substituents.
In conclusion, we previously achieved a stabilization of the elusive ditopic Lewis acid 1,2:1,2-bis(2,2′-biphenylylene)diborane(4) through coordination of one H− ligand, which ends up in a B⋅⋅⋅B-bridging position ([2H]−).23 As a second effect, which has been studied herein, the bonded hydride ion causes an “Umpolung” of the still largely exposed B−B core, which gains an electron-donating, nucleophilic character: The Lewis acidic 9-H-9-borafluorene (HBFlu) adds to [2H]− with formation of the B3 cluster [3]−—even in the Lewis basic solvent THF. An attack of [2H]− on the electrophiles MeI or Me3SiCl leads to the formation of B−C (see 4) or B−Si bonds (see 6), accompanied by 1,2-phenyl shifts in the initial boron-doped dibenzo[g,p]chrysene scaffold. An unleashed [:BFlu]− intermediate is also the reactive species in the [4+1] cycloaddition of [2H]− with 2,3-dimethyl-1,3-butadiene, which produces the spiroborate [7]−. Such application of a boryl anion as reported here for [:BFlu]− is so far unprecedented and of considerable synthetic value.
Acknowledgements
T.T. thanks the Fonds der Chemischen Industrie for a Ph.D. grant. We thank Albemarle Germany GmbH (Frankfurt) for the generous gift of chemicals.
Conflict of interest
The authors declare no conflict of interest.




