Energy-Efficient Solar Photochemistry with Luminescent Solar Concentrator Based Photomicroreactors
Abstract
The sun is the most sustainable light source available on our planet, therefore the direct use of sunlight for photochemistry is extremely appealing. Demonstrated here, for the first time, is that a diverse set of photon-driven transformations can be efficiently powered by solar irradiation with the use of solvent-resistant and cheap luminescent solar concentrator based photomicroreactors. Blue, green, and red reactors can accommodate both homogeneous and multiphase reaction conditions, including photochemical oxidations, photocatalytic trifluoromethylation chemistry, and metallaphotoredox transformations, thus spanning applications over the entire visible-light spectrum. To further illustrate the efficacy of these novel solar reactors, medicinally relevant molecules, such as ascaridole and an intermediate of artemisinin, were prepared as well.
Introduction
Solar-energy-conversion technologies are extremely attractive because of the unrivaled potential of this energy source.1 To date, the majority of research attention has focused on solar electricity,2 solar thermal,3 and solar fuels,4 while the synthesis of chemicals powered by solar light has gathered significantly less scrutiny. However, the production of fine chemicals, including drugs and fragrances, could significantly benefit from green processes directly powered by solar energy.5, 6
In the last decade, the field of photochemistry has been revolutionized by the introduction of photoredox catalysis, which vastly expanded the scope of reactions promoted by light irradiation.7-9 Photoredox reactions are ideal candidates for solar applications since the use of photocatalysts with high extinction coefficients makes them good photon harvesters. Moreover, the majority of the synthetically useful photocatalysts absorb in the visible range, which is the region of peak intensity in the solar spectrum.10, 11 Despite the attractiveness of green and sustainable solar photochemistry, its low popularity6 can be attributed to the broad spectral distribution, the fluctuations in solar irradiance, and the dilute energy content (up to 6.6 mol m−2⋅h−1).10, 11 These shortcomings limit the usefulness of solar energy in photochemical transformations and result in impractical, long reaction times for solar-powered photocatalysis.
Here, we show that, with a dedicated reactor design based on the luminescent solar concentrator concept, it is possible to surmount these limitations, fully unleashing the sustainable potential of solar photochemistry. Luminescent solar concentrators (LSCs) are inexpensive slabs of luminophore-doped polymeric materials that harvest, down-convert, and concentrate solar photons.12, 13 LSCs can be used as photon harvesters for photochemical reactions in so-called luminescent solar concentrator-photomicroreactors (LSC-PMs).14 In LSC-PMs, the LSC lightguide is embedded with microreactor channels, acting as final absorbers of the sunlight-generated luminescent photons. Our original LSC-PM implementation was characterized by limited chemical and optical stability, which prevented the applicability of LSC-PMs to a majority of the reported visible light-driven photochemical reactions. Herein, a conceptually new design is presented for the integration of LSCs and microreactors that eliminates these shortcomings. Moreover, our microreactor can concentrate both direct and diffuse solar photons, and can be coupled with light sensors positioned at the device's edge. When combined in a feedback loop, this cheap self-optimization system can autonomously compensate for the fluctuations in solar irradiance, for example, for passing clouds.15 Hence, we provide a blueprint to carry out essentially every visible-light-driven photochemical transformation in the sun.
Results and Discussion
Our novel design uses perfluoroalkoxy alkane (PFA) capillaries embedded in poly(methyl methacrylate) (PMMA) lightguides (Figure 1 A). This design offers a substantial photon-flux improvement compared to the original PDMS-based design because of the material's higher refractive index. Consequently, 40 % more photons are directed to the reaction channels, leading to substantial rate accelerations (see Figure S12 in the Supporting Information). The adoption of PMMA as a material for the LSC-PM also came with additional benefits in terms of cost-effectiveness, commercial availability of the polymeric slabs, and ease in reactor scaling (10×10, 20×20, and 30×30 cm2, from 0.18 to 1.59 mL; Figure 1 C). Moreover, PMMA provides a slower luminophore degradation (up to 3 % decrease in absorbance over 2 years)16 and is compatible with a great variety of luminophore dopants. The latter aspect paves the way to construct red, green, and blue LSC-PM devices, hence providing access to all visible-light-mediated transformations, for example, methylene blue for the red device, eosin Y and rose Bengal for the green, and Ru-based metal complexes for the blue reactor (Figures 1 B and C).
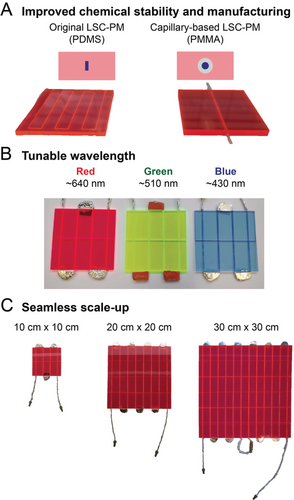
A) Photographs and schematic comparison between the capillary-based LSC-PM (right) and the original design (left). B) Photographs of LSC-PMs with different dye dopants. C) LSC-PMs of different sizes, with irradiated volumes of 177 μL, 707 μL, and 1.59 mL respectively.
To test the generality of our next-generation LSC-PM, we carried out a diverse set of photocatalytic transformations that have shown great potential in synthetic organic chemistry (Figure 2). The first photocatalytic reaction, carried out in a red LSC-PM, was the hydroxylation of aryl boronic acids. While the reaction was originally reported by Xiao and co-workers with Ru(bpy)32+, Scaiano et al. showed that methylene blue was equally effective.17, 18 We commenced by adapting the reaction to continuous-flow, using phenylboronic acid as a model substrate. The oxygen balloon employed in the original literature report for a batch reaction was replaced by a pure oxygen stream, resulting in a gas/liquid slug flow regime. The adoption of a slug flow regime increases the mass transfer between the gas and the liquid phase and results in an acceleration of the apparent reaction kinetics.19 Ideally, this acceleration can extend the point where the light input becomes a limiting factor, thus providing the maximum attainable productivity under solar conditions. Indeed, the reaction was accelerated from the 6 hours required in the original report to only 5 minutes in the LSC-PM under AM 1.5G solar conditions. Notably, the reaction kinetics were now partly light-limited, as shown by the fact that the LSC-PM provided a substantial acceleration compared to a simple capillary irradiated under similar conditions (Figure 2 A). While the observed acceleration by a factor of two is impressive, it does not compare to the fivefold acceleration predicted by the Monte Carlo simulations for the device (see the Supporting Information). We suspected that the reaction kinetics were not linearly correlated with the light input at one sun intensity for this specific transformation. To verify this hypothesis, we switched to a singlet-oxygen-mediated reaction. It is known that the photosensitized production of singlet oxygen is linearly dependent on the incident photon flux.14 In particular, the oxidation of methionine thioether to the corresponding sulfoxide was chosen, given that this product has attracted considerable attention over the past few years.20, 21 In this case, the LSC-PM provided a sixfold acceleration under solar simulated conditions (Figure 2 B; see the Supporting Information).
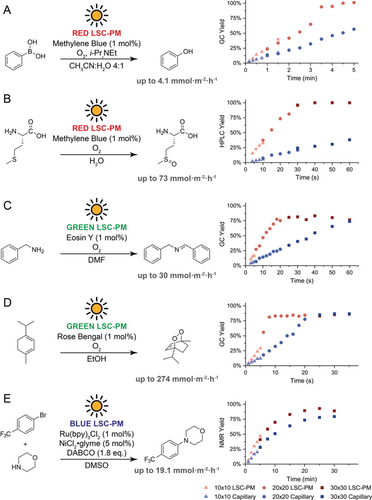
Overview of the reactions performed in the LSC-PM. A) Hydroxylation of boronic acids. B) Oxidation of (l)-methionine. C) Benzylamine oxidation. D) α-Terpinene oxidation. E) Morpholine arylation.
As a direct consequence of the down-converting nature of LSCs, the lower the energy of the luminescent photons (i.e., red-shifted photons), the higher the overall optical efficiency will be. However, there are far more photocatalysts absorbing in the green and blue portions of the visible spectrum than in the red region. For this reason, an organic fluorescent dye emitting at 530 nm, that is, DFSB-K160, was selected to manufacture green LSC-PMs for reactions catalyzed by, for example, eosin Y and rose bengal. The calculated LSC-PM photon-flux accelerations compared to simple capillaries were found to be around 4.5-fold for both photocatalysts (see the Supporting Information). The combination of eosin Y and the green LSC-PM was investigated for the eosin Y-mediated aerobic oxidation of benzylamine to dibenzylimine (Figure 2 C).22 A 3.3-fold acceleration was observed in the LSC-PM (see the Supporting Information), producing the imine in about 75 % yield at 30 mmol m−2 h−1. Next, to test the combination of LSC-PM and rose bengal, we adopted the well-known cycloaddition of singlet oxygen to α-terpinene, yielding the anthelmintic drug ascaridole. Notably, this reaction was the first photochemical reaction performed in a solar-driven manufacturing plant by Günther Otto Schenck in 1948.23 Because of its efficiency, this reaction is often used as benchmark for singlet oxygen reaction systems.6, 24 Under solar-simulated irradiation, a productivity of 274 mmol m−2 h−1 of solar irradiated surface was observed (Figure 2 D).11 This high productivity emphasizes the validity of the LSC-PM design for photon-efficient solar photochemistry.
LSC-PMs capable of concentrating blue photons are even more appealing to match the absorption (ca. 450 nm range) of several commonly used photocatalysts, including Ru(bpy)3Cl2, Mes-Acr+, and 2-CzPN. The frontier of photoredox chemistry is currently exemplified by the so-called dual-catalysis concept, also known as metallaphotoredox catalysis, which constitutes a synergetic merger of transition-metal catalysis and photoredox catalysis.27 Metallaphotoredox provides access to activation modes that are both unique and complementary to traditional metal catalysis, naturally attracting significant interest. Despite the novelty of this field, some reactions are already characterized by efficient catalyst turnover and photon-limited kinetics, thus constituting an interesting application benchmark for the LSC-PM. To explore the applicability of metallaphotoredox reactions in the LSC-PM, we chose the aryl amination catalyzed by NiCl2⋅glyme and Ru(bpy)3Cl2 as reported by Buchwald, MacMillan, and co-workers.28, 29 The reaction conditions were adapted to flow using DMSO as a solvent, instead of DMA, to obtain a homogeneous reaction mixture, and p-bromobenzotrifluoride was selected as a model substrate. The reaction was performed in the blue LSC-PM, resulting in a slight acceleration compared to the nondoped analogue (Figure 2 E). What is remarkable is that the selectivity of this reaction is improved, with a maximum yield in the LSC-PM of 93 % versus a maximum yield in the capillary of 80 %. This effect can be attributed to the decreased UV content of the light reaching the reaction channels in the LSC-PM.30
Since the primary application of the LSC-PM is in solar photochemistry, we performed some reactions with natural sunlight. Because of its simplicity and safety, the first reaction selected for outdoor testing on a larger scale was the photooxidation of methionine (Figure 3). In this scenario, a cartridge filled with activated charcoal was placed at the reactor outlet to remove the photocatalyst from the reaction stream and thus quench the reaction in-line. Consequently, the outlet contained only a water mixture of unreacted starting material and product. The LSC-PM reactor showed consistently higher reaction yields compared with that of a simple capillary (Figure 3 A). This increase can be attributed to the increased photon harvesting ability of the LSC-PM leading to higher photon fluxes in the reaction channels.
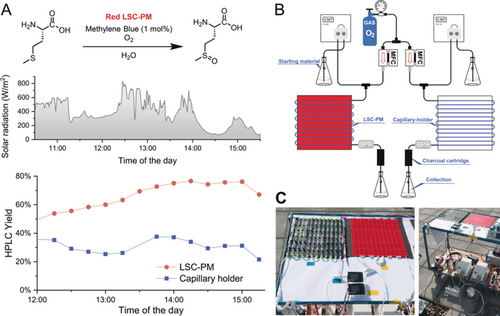
Outdoor experiment for the methylene-blue-catalyzed photooxidation of methionine. A) Reaction scheme, solar irradiation, and yield over time. B) Flow scheme of the experimental setup. C) Photographs of the experimental setup.
Recently, we showed that the light intensity measured at the edge of the device can be used to dynamically adjust the residence time in the reactor, thus autonomously correcting for the natural fluctuations in solar irradiance.15 We applied this approach for the light-limited Ru(bpy)32+-catalyzed trifluoromethylation using trifluoroacetic anhydride reported by Stephenson and co-workers.25, 26 In this case, little to no acceleration was observed with the use of the blue LSC-PM as opposed to a simple capillary reactor, likely because of the limited light-collecting efficiency of the blue dye. In fact, to generate fluorescent blue photons, UV light is needed, but the abundance in the solar spectrum is limited. However, the developed system has additional advantages compared to a simple capillary reactor as a result of its potential for automation. After measuring the apparent reaction kinetics at difference irradiance intensities, we tested an automated reaction control system for the solar-powered trifluoromethylation of mesitylene. The reaction was performed outdoors during a partially sunny winter day with substantial fluctuation in solar irradiance intensity. As shown in Figure 4, the reaction control system compensated for the fluctuations in solar irradiance in real time by changing the pump flow rate and afforded relatively stable reaction yields at about 45 % with 96 % selectivity.25
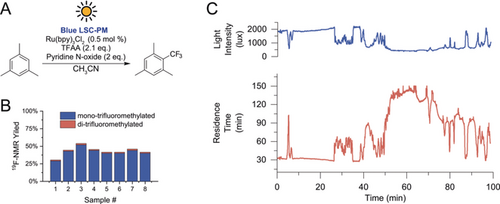
Outdoor trifluoromethylation of mesitylene in the blue LSC-PM. A) Reaction conditions. B) Reaction yield of the samples collected during the experiment. The first sample was taken after 60 minutes, and after that, one sample every 5 minutes was acquired. C) Light intensity measured at the device edge and proportional changes in residence time during the experiment operated by the autonomous reaction control system.
All examples showcase the relative simplicity in adapting photochemical reactions for solar applications using the LSC-PM. However, as highlighted in the introduction, the productivity afforded by solar photochemistry has a physical limit of several moles per square meter per hour of solar irradiated area.6 This means that solar photochemistry is especially a viable option for high added-value chemicals such as drugs and fragrances. Therefore, to provide a realistic application for the LSC-PM concept, the continuous solar-driven synthesis of artemisinin31 was taken as an example. Artemisinin and its derivatives are the most effective drugs against malaria, and their yearly production is not sufficient to meet the world's needs.32, 33 A solar-based production plant would be particularly suited for those limited resource settings where the disease is endemic. The crucial step in the semisynthetic approach is the biomimetic photooxygenation of dihydroartemisinic acid to the corresponding endoperoxide, which yields, after acid-catalyzed Hock cleavage, artemisinin (Figure 5, top).
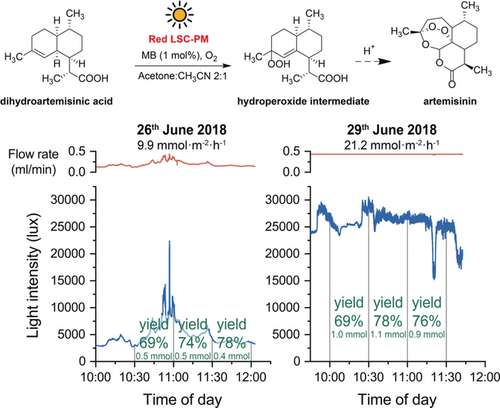
Methylene-blue-catalyzed photooxidation of dihydroartemisinic acid under natural sunlight irradiation in a red LSC-PM. The experiments were performed in the Max Plank Institute for Colloids and Interfaces of Potsdam (Germany) on the 26th and 29th of June 2018, under different sky conditions (cloudy in the first case, and sunny in the second). The different irradiance received by the reactor resulted in vastly different solar productivities but with similar yields thanks to the reaction control system.
The published reaction conditions were adapted for the use of methylene blue as a photocatalyst and, after having acquired the kinetic profile, the reaction was performed on a window ledge, under solar irradiation (see the Supporting Information) with a reaction control system. In Figure 5 the results from the solar experiments are presented. The use of the reaction control system resulted in a similar reaction yield (between 69 and 78 %), with a significantly higher productivity during the second day (up to 21.2 mmol m−2 h−1) because of the higher irradiance.
Conclusion
In 1912, Giacomo Ciamician challenged the scientific community to imagine a chemical industry run on solar energy.34 Inspired by Ciamician's grand vision, we have developed a novel reactor design that constitutes a versatile, inexpensive, and photon-efficient solution to harvest solar energy for synthetic applications.35 Several different photon-driven reactions are showcased, ranging from photochemical oxidations to metallaphotoredox couplings. With the adoption of a reaction control system, stable product quality can be ensured even during fluctuating irradiance conditions. Notably, the LSC-PM design reported here is mostly transparent to IR radiation, and avoids heating of the reactor. This feature also means that a further increase in the fraction of utilizable solar light can be achieved by combining it with other solar-energy conversion technologies capable of making productive use of such low-energy photons, like photovoltaic cells.36
Acknowledgements
D.C. and T.N. would like to acknowledge the European Union for a Marie Curie ITN Grant (Photo4Future, grant number 641861). P.R. received a Marie Curie European post-doctoral fellowship (MOSPhotocat, Grant No. 793677). K.G. and P.H.S. thanks for generous financial support provided by the Max Planck Society and DFG InCHeM (FOR 2177).
Conflict of interest
The authors declare no conflict of interest.




