Construction of Hexahydrophenanthrenes By Rhodium(I)-Catalyzed Cycloisomerization of Benzylallene-Substituted Internal Alkynes through C−H Activation
Yasuaki Kawaguchi
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorDr. Shigeo Yasuda
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Chisato Mukai
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorYasuaki Kawaguchi
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorDr. Shigeo Yasuda
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Chisato Mukai
Division of Pharmaceutical Sciences, Graduate School of Medical Sciences, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorAbstract
The treatment of benzylallene-substituted internal alkynes with [RhCl(CO)2]2 effects a novel cycloisomerization by C(sp2)−H bond activation to produce hexahydrophenanthrene derivatives. The reaction likely proceeds through consecutive formation of a rhodabicyclo[4.3.0] intermediate, σ-bond metathesis between the C(sp2)−H bond on the benzene ring and the C(sp2)−RhIII bond, and isomerization between three σ-, π-, and σ-allylrhodium(III) species, which was proposed based on experiments with deuterated substrates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange201605640-sup-0001-misc_information.pdf16.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent reviews, see:
- 1aC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292;
- 1bL. Ackermann, Chem. Rev. 2011, 111, 1315–1345;
- 1cL. McMurray, F. O'Hara, M. J. Gaunt, Chem. Soc. Rev. 2011, 40, 1885–1898;
- 1dJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960–9009; Angew. Chem. 2012, 124, 9092–9142;
- 1eJ. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369–375.
- 2For selected recent reviews, see:
- 2aM. Murakami, T. Matsuda, Chem. Commun. 2011, 47, 1100–1105;
- 2bC. Aïssa, Synthesis 2011, 3389–3407;
- 2cL. Souillart, N. Cramer, Chem. Rev. 2015, 115, 9410–9464.
- 3C. Mukai, Y. Ohta, Y. Oura, Y. Kawaguchi, F. Inagaki, J. Am. Chem. Soc. 2012, 134, 19580–19583.
- 4Similar cycloadditions of allenylcyclopropane- and allenylcyclobutane-alkynes have also been described; see:
- 4aF. Inagaki, K. Sugikubo, Y. Miyashita, C. Mukai, Angew. Chem. Int. Ed. 2010, 49, 2206–2210; Angew. Chem. 2010, 122, 2252–2256;
- 4bF. Inagaki, K. Sugikubo, Y. Oura, C. Mukai, Chem. Eur. J. 2011, 17, 9062–9065.
- 5The mechanism of the reaction of allenylcyclopentane-alkynes 1 has also been investigated by DFT calculations by another group. The postulated mechanism is similar to ours, although there is a slight difference; see: G. Huang, J. Org. Chem. 2015, 80, 7564–7571.
- 6S. Kaarsemaker, J. Coops, Recl. Trav. Chim. Pays-Bas 1952, 71, 261–276.
- 7For the allenylcyclobutane derivatives, the reaction would proceed via a rhodabicyclo[4.3.0] intermediate similar to 4, which could accelerate the cleavage of the unfunctionalized cyclobutane C−C bond by relief of the high ring-strain energy (26.3 kcal mol−1); see Refs. [4b, 6].
- 8For the allenylcyclopropane derivatives, we assume that a six-membered rhodacyclic intermediate is initially formed because cyclopropane has a fairly high ring-strain energy (27.5 kcal mol−1): see Ref. [4a] and:
- 8aJ. W. Knowlton, F. D. Rossini, J. Res. Natl. Bur. Stand. 1949, 43, 113–115; a similar rhodacyclic intermediate could be considered for the reaction of allenylcyclopropane-alkenes; see:
- 8bK. Sugikubo, F. Omachi, Y. Miyanaga, F. Inagaki, C. Matsumoto, C. Mukai, Angew. Chem. Int. Ed. 2013, 52, 11369–11372; Angew. Chem. 2013, 125, 11579–11582.
- 9For selected examples of RhI-catalyzed cycloisomerizations or cycloadditions of allene-alkyne substrates, see:
- 9aK. M. Brummond, H. Chen, P. Sill, L. You, J. Am. Chem. Soc. 2002, 124, 15186–15187;
- 9bX. Jiang, S. Ma, J. Am. Chem. Soc. 2007, 129, 11600–11607;
- 9cY. Oonishi, A. Hosotani, Y. Sato, J. Am. Chem. Soc. 2011, 133, 10386–10389;
- 9dY. Oonishi, Y. Kitano, Y. Sato, Angew. Chem. Int. Ed. 2012, 51, 7305–7308; Angew. Chem. 2012, 124, 7417–7420;
- 9eY. Oonishi, T. Yokoe, A. Hosotani, Y. Sato, Angew. Chem. Int. Ed. 2014, 53, 1135–1139; Angew. Chem. 2014, 126, 1153–1157.
- 10Y. Kawaguchi, S. Yasuda, A. Kaneko, Y. Oura, C. Mukai, Angew. Chem. Int. Ed. 2014, 53, 7608–7612; Angew. Chem. 2014, 126, 7738–7742.
- 11The stereochemistry of the exocyclic double bond in the cyclized products 7 was determined to be as shown by NOE experiments (see the Supporting Information for details).
- 12For selected examples of RhI-catalyzed carbonylative [2+2+1] cycloadditions of allene-alkyne substrates, see:
- 12aK. M. Brummond, D. Chen, Org. Lett. 2008, 10, 705–708;
- 12bF. Inagaki, S. Kitagaki, C. Mukai, Synlett 2011, 594–614, and references therein. For selected examples of RhI-catalyzed carbonylative [2+2+1] cycloadditions of other allene substrates, see:
- 12cF. Inagaki, S. Narita, T. Hasegawa, S. Kitagaki, C. Mukai, Angew. Chem. Int. Ed. 2009, 48, 2007–2011; Angew. Chem. 2009, 121, 2041–2045;
- 12dT. Iwata, F. Inagaki, C. Mukai, Angew. Chem. Int. Ed. 2013, 52, 11138–11142; Angew. Chem. 2013, 125, 11344–11348.
- 13The reason for the improvement of the yield of 7 a is uncertain at this stage. Initiation of the reaction at the boiling point of toluene or xylene might presumably help the rapid extrusion of CO from the RhI catalyst, thereby improving the yield of 7 a and suppressing the formation of 8 a.
- 14The yields of 7 f, 12 f, 12 g, and 18 were determined by 1H NMR analysis because these compounds could not be isolated in pure form by column chromatography.
- 15M. E. Jung, G. Piizzi, Chem. Rev. 2005, 105, 1735–1766.
- 16For selected reviews on the transformation of organosilicon compounds, see:
- 16aE. Colvin, Silicon Reagents in Organic Synthesis, Academic Press, San Diego, 1998;
- 16bT. Hiyama, E. Shirakawa, Top. Curr. Chem. 2002, 219, 61–85; for selected examples of the transformation of alkenyl silanes using TFA, see:
- 16cR. Morita, E. Shirakawa, T. Tsuchimoto, Y. Kawakami, Org. Biomol. Chem. 2005, 3, 1263–1268;
- 16dB. M. Trost, M. R. Machacek, B. D. Faulk, J. Am. Chem. Soc. 2006, 128, 6745–6754.
- 17During the transformation of 7 into 10, desilylation seemed to occur prior to isomerization of the double bond because no reaction took place when 9 was treated with 20 equiv of TFA at 60 °C for 24 h.
- 18X-ray analysis of 12 h unambiguously established its 5-alkylidenetricyclo[8.4.0.04, 9]tetradecatetraene structure (see the Supporting Information for details).
- 19We also attempted the construction of larger tricyclic frameworks by the reaction of the one-carbon-homologated substrate 20. However, no reaction occurred under the standard conditions.
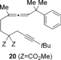
- 20
- 20aC. Mukai, F. Inagaki, T. Yoshida, K. Yoshitani, Y. Hara, S. Kitagaki, J. Org. Chem. 2005, 70, 7159–7171;
- 20bC. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 2011, 111, 1954–1993.
- 21For examples of cyclizations involving σ-bond metathesis between C−H and C−Rh bonds, see:
- 21aL. Souillart, N. Cramer, Chem. Sci. 2014, 5, 837–840;
- 21bK. Masutomi, K. Noguchi, K. Tanaka, J. Am. Chem. Soc. 2014, 136, 7627–7630;
- 21cT. Matsuda, I. Yuihara, Chem. Commun. 2015, 51, 7393–7396, and Ref. [9d].
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




