Electrical Network of Single-Crystalline Metal Oxide Nanoclusters Wired by π-Molecules†
Corresponding Author
Dr. Ryo Tsunashima
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)Search for more papers by this authorYoshifumi Iwamoto
Department of Biology and Chemistry, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorYusuke Baba
Department of Biology and Chemistry, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorChisato Kato
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Search for more papers by this authorKatsuya Ichihashi
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Search for more papers by this authorDr. Sadafumi Nishihara
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Institute for Advanced Materials Research, Hiroshima University, Higashi-Hiroshima 739-8530 (Japan)
Search for more papers by this authorProf. Katsuya Inoue
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Institute for Advanced Materials Research, Hiroshima University, Higashi-Hiroshima 739-8530 (Japan)
Search for more papers by this authorProf. Katsuya Ishiguro
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorProf. Yu-Fei Song
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, 100029 Beijing (P. R. China)
Search for more papers by this authorProf. Tomoyuki Akutagawa
Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Sendai 980-8577 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Ryo Tsunashima
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)Search for more papers by this authorYoshifumi Iwamoto
Department of Biology and Chemistry, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorYusuke Baba
Department of Biology and Chemistry, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorChisato Kato
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Search for more papers by this authorKatsuya Ichihashi
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Search for more papers by this authorDr. Sadafumi Nishihara
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Institute for Advanced Materials Research, Hiroshima University, Higashi-Hiroshima 739-8530 (Japan)
Search for more papers by this authorProf. Katsuya Inoue
Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526 (Japan)
Institute for Advanced Materials Research, Hiroshima University, Higashi-Hiroshima 739-8530 (Japan)
Search for more papers by this authorProf. Katsuya Ishiguro
Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi, 753 8512 (Japan)
Search for more papers by this authorProf. Yu-Fei Song
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, 100029 Beijing (P. R. China)
Search for more papers by this authorProf. Tomoyuki Akutagawa
Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Sendai 980-8577 (Japan)
Search for more papers by this authorThis work was partly supported by the cooperative research program of the Network Joint Research Centre for Materials and Devices of Japan and Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abstract
In a mixed-valence polyoxometalate, electrons are usually delocalized within the cluster anion because of low level of inter-cluster interaction. Herein, we report the structure and electrical properties of a single crystal in which mixed-valence polyoxometalates were electrically wired by cationic π-molecules of tetrathiafulvalene substituted with pyridinium. Electron-transport characteristics are suggested to represent electron hopping through strong interactions between cluster and cationic π-molecules.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201406223_sm_miscellaneous_information.pdf606.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. F. Song, R. Tsunashima, Chem. Soc. Rev. 2012, 41, 7384–7402;
- 1bD.-L. Long, R. Tsunashima, L. Cronin, Angew. Chem. 2010, 122, 1780–1803; Angew. Chem. Int. Ed. 2010, 49, 1736–1758;
- 1cA. Müller, F. Peters, M. T. Pope, D. Gatteschi, Chem. Rev. 1998, 98, 239–272;
- 1dC. L. Hill, Chem. Rev. 1998, 98, 1–2;
- 1eP. Kögerler, B. Tsukerblat, A. Müller, Dalton Trans. 2010, 39, 21–36;
- 1fU. Kortz, A. Müller, J. Slageren, J. Schnack, N. S. Dalal, M. Dressel, Coord. Chem. Rev. 2009, 253, 2315–2327;
- 1gN. Mizuno, M. Misono, Chem. Rev. 1998, 98, 199–218.
- 2
- 2aJ. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, Chem. Soc. Rev. 2012, 41, 7464–7478;
- 2bN. Casañ-Pastor, L. C. Baker, J. Am. Chem. Soc. 1992, 114, 10384–10401;
- 2cR. A. Prados, M. T. Pope, Inorg. Chem. 1976, 15, 2547–2553;
- 2dC. Sanchez, J. Livage, J. P. Launay, M. Fournier, J. Am. Chem. Soc. 1983, 105, 6817–6823.
- 3J. Lehmann, A. Gaita-Ariño, E. Coronado, D. Loss, Nat. Nanotechnol. 2007, 2, 312–317.
- 4C. Fleming, D.-L. Long, N. McMillan, J. Johnson, N. Bovet, V. Dhanak, N. Gadegaard, P. Kögerler, L. Cronin, M. Kadodwala, Nat. Nanotechnol. 2008, 3, 229–233.
- 5
- 5aD. Velessiotis, N. Glezos, V. Ioannou-Sougleridis, J. Appl. Phys. 2005, 98, 0845031–0845034;
- 5bA. M. Douvas, E. Makarona, N. Glezos, P. Argitis, J. A. Mielczarski, E. Mielczarski, ACS Nano 2008, 2, 733–742.
- 6
- 6aG. Saito, Y. Yoshida, Bull. Chem. Soc. Jpn. 2007, 80, 1–137;
- 6bT. Akutagawa, T. Ohta, T. Hasegawa, T. Nakamura, C. A. Christensen, J. Becher, Proc. Natl. Acad. Sci. USA 2002, 99, 5028–5033;
- 6cR. Tsunashima, Y. Noda, Y. Tatewaki, S.-i. Noro, T. Akutagawa, T. Nakamura, T. Matsumoto, T. Kawai, Appl. Phys. Lett. 2008, 93, 1731021–17310213;
- 6dE. Coronado, C. J. Gómez-García, Chem. Rev. 1998, 98, 273–296;
- 6eL. Ouahab, M. Bencharif, D. Grandjean, C. R. Acad. Sci. Ser. II 1988, 307, 749–755;
- 6fS. Triki, L. Ouahab, J.-F. Halet, O. Peña, J. Padiou, D. Grandjean, C. Garrigou-Lagrange, P. Delhaes, J. Chem. Soc. Dalton Trans. 1992, 1217–1227;
- 6gE. Coronado, C. Giménez-Saiz, C. J. Gómez-García, Coord. Chem. Rev. 2005, 249, 1776–1796;
- 6hJ. R. Galán-Mascarós, C. Giménez-Saiz, S. Triki, C. J. Gómez-García, E. Coronado, L. Ouahab, Angew. Chem. Int. Ed. Engl. 1995, 34, 1460–1462; Angew. Chem. 1995, 107, 1601–1603.
- 7
- 7aB. A. Scott, S. J. La Placa, J. B. Torrance, B. D. Silverman, B. Welber, J. Am. Chem. Soc. 1977, 99, 6631–6639;
- 7bT. Mori, A. Kobayashi, Y. Sasaki, H. Kobayashi, G. Saito, H. Inokuchi, Bull. Chem. Soc. Jpn. 1984, 57, 627–633.
- 8S. C. Lee, A. Ueda, H. Kamo, K. Takahashi, M. Uruichi, K. Yamamoto, K. Yakushi, A. Nakao, R. Kumai, K. Kobayashi, H. Nakao, Y. Murakami, H. Mori, Chem. Commun. 2012, 48, 8673–8675.
- 9R. Bozio, I. Zanon, A. Girlando, C. Pecile, J. Chem. Phys. 1979, 71, 2282–2293.
- 10Similar orbital energies between TTFPy and [PMoVI12O40]3− are only expected between the HOMO of TTFPy and the LUMO of the cluster anion. The redox potentials of these orbitals in the solution phase show the LUMO of [PMoVI12O40]3− is higher than HOMO of TTFPy by ca. 0.2 eV, however these two orbitals do not overlap (see Supporting Information). The HOMO–LUMO gap of [PMoVI12O40]3− is observed at 232 or 318 nm. Namely, the orbital energies of the O atoms are 4–5 eV below the HOMO of TTFPy.
- 11J. Han, M. Shen, W. Cao, A. M. R. Senos, P. Q. Mantas, Appl. Phys. Lett. 2003, 82, 67–69.
- 12
- 12aD. Yu, C. Wang, B. L. Wehrenberg, P. Guyot-Sionnest, Phys. Rev. Lett. 2004, 92, 2168021–2168024;
- 12bR. Parthasarathy, X.-M. Lin, H. M. Jaeger, Phys. Rev. Lett. 2001, 87, 1868071–1868074;
- 12cT. B. Tran, I. S. Beloborodov, X. M. Lin, T. P. Bigioni, V. M. Vinokur, H. M. Jaeger, Phys. Rev. Lett. 2005, 95, 0768061–07680614;
- 12dY. Tatewaki, Y. Noda, T. Akutagawa, R. Tsunashima, S.-i. Noro, T. Nakamura, H. Hasegawa, S. Mashiko, J. Becher, J. Phys. Chem. C 2007, 111, 18871–18877;
- 12eY. Noda, S.-i. Noro, T. Akutagawa, T. Nakamura, Phys. Rev. Lett. 2010, 82, 2054201–2054206;
- 12fT. Sugawara, M. Minamoto, M. M. Matsushita, P. Nickels, S. Komiyama, Phys. Rev. B 2008, 77, 2353161–2353167;
- 12gJ. M. Wessels, H.-G. Nothofer, W. E. Ford, F. Wrochem, F. Scholz, T. Vossmeyer, A. Schroedter, H. Weller, A. Yasuda, J. Am. Chem. Soc. 2004, 126, 3349–3356;
- 12hR. Parthasarathy, X.-M. Lin, H. M. Jaeger, Phys. Rev. Lett. 2001, 87, 1868071–1868074.
- 13G. Orsini, V. Tricoli, J. Mater. Chem. 2011, 21, 14530–14542.
- 14L. Wang, B. Zhang, J. Zhang, Inorg. Chem. 2006, 45, 6860–6863.
- 15
- 15aA band was observed at 772 nm (in CH2Cl2/CH3CN) upon both oxidation and protonation of a similar compound of 2,3-dimethylthio-6-pyridyl-tetrathiafulvalene, see Ref. [15b];
- 15bQ. Y. Zhu, Y. Liu, W. Lu, Y. Zhang, G. Q. Bian, G. Y. Niu, J. Dai, Inorg. Chem. 2007, 46, 10065–10070.
- 16
- 16a[P MoVMoVI11O40]4− in acetonitrile shows bands at 342 and 750 nm with a shoulder at 1050 nm, see Ref [16b];
- 16bH.-R. Sun, S.-Y. Zhang, J.-Q. Xu, G.-Y. Yang, T.-S. Shi, J. Electroanal. Chem. 1998, 455, 57–68.
- 17(TTFPyH)2[PMoVMoVI11O40]⋅n H2O (1): C22H16Mo12N2O46PS8 for n=6, Mr=2483.18 g mol−1, plate like crystal, 0.106×0.134×0.170 mm3, T=173(2) K, triclinic, space group
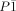 , a=11.663(11), b=11.750(12), c=12.304(10) Å, α=61.81(4), β=70.33(4), γ=78.41(6)°, V=1398(2) Å3, Z=1, ρ=2.950 g cm−3, F(000)=1177, 11445 reflections measured, 4895 unique (Rint=0.0530), 430 refined parameters, R1=0.0570, wR2=0.1497. Six numbers of crystalline water molecules were crystallographically identified and their six number of O atoms were included to crystallographic composition shown above. CCDC 1008211 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, a=11.663(11), b=11.750(12), c=12.304(10) Å, α=61.81(4), β=70.33(4), γ=78.41(6)°, V=1398(2) Å3, Z=1, ρ=2.950 g cm−3, F(000)=1177, 11445 reflections measured, 4895 unique (Rint=0.0530), 430 refined parameters, R1=0.0570, wR2=0.1497. Six numbers of crystalline water molecules were crystallographically identified and their six number of O atoms were included to crystallographic composition shown above. CCDC 1008211 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




