An Alternative to the Classical α-Arylation: The Transfer of an Intact 2-Iodoaryl from ArI(O2CCF3)2†
Dr. Zhiyu Jia
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorDr. Erik Gálvez
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorProf. Dr. Rosa María Sebastián
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorProf. Dr. Roser Pleixats
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorDr. Ángel Álvarez-Larena
Servei de Difracció de Raigs X, Universitat Autònoma de Barcelona
Search for more papers by this authorDr. Eddy Martin
Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. Adelina Vallribera
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Adelina Vallribera, Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Alexandr Shafir, Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Dr. Alexandr Shafir
Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Adelina Vallribera, Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Alexandr Shafir, Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorDr. Zhiyu Jia
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorDr. Erik Gálvez
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorProf. Dr. Rosa María Sebastián
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorProf. Dr. Roser Pleixats
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Search for more papers by this authorDr. Ángel Álvarez-Larena
Servei de Difracció de Raigs X, Universitat Autònoma de Barcelona
Search for more papers by this authorDr. Eddy Martin
Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. Adelina Vallribera
Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Adelina Vallribera, Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Alexandr Shafir, Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Dr. Alexandr Shafir
Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Adelina Vallribera, Department of Chemistry and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universitat Autònoma de Barcelona, Bellaterra (Spain)
Alexandr Shafir, Institute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorThis work was supported by the ICIQ, MICINN (CTQ2011-22649), the MEC (Cons. Ing. CSD2007-00006), the Generalitat de Catalunya (2014SGR1192 and 2014SGR1105), and the China Scholarship Council (fellowship to Z.J.). We thank MINECO for support through grant CTQ2013-46705-R and Severo Ochoa Excellence Accreditation 2014–2018 (SEV-2013-0319).
Abstract
The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ3-iodanes. Here, we describe an alternative metal-free α-arylation using ArI(O2CCF3)2 as the source of a 2-iodoaryl group. The reaction is applicable to activated ketones, such as α-cyanoketones, and works with substituted aryliodanes. This formal CH functionalization reaction is thought to proceed through a [3,3] rearrangement of an iodonium enolate. The final α-(2-iodoaryl)ketones are versatile synthetic building blocks.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201405982_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1997, 119, 11108–11109;
- 1bJ. M. Fox, X. Huang, A. Chieffi, S. L. Buchwald, J. Am. Chem. Soc. 2000, 122, 1360–1370;
- 1cB. C. Hamann, J. F. Hartwig, J. Am. Chem. Soc. 1997, 119, 12382–12383.
- 2For a review on metal-catalyzed α-arylation, see:
- 2aC. C. C. Johansson, T. J. Colacot, Angew. Chem. Int. Ed. 2010, 49, 676–707; Angew. Chem. 2010, 122, 686–718;
- 2bF. Bellina, R. Rossi, Chem. Rev. 2010, 110, 1082—1146.
- 3
- 3aF. M. Beringer, P. S. Forgione, J. Org. Chem. 1963, 28, 714–717;
- 3bF. M. Beringer, W. J. Daniel, S. A. Galton, G. Rubin, J. Org. Chem. 1966, 31, 4315–4318;
- 3cC. H. Oh, J. S. Kim, H. H. Jung, J. Org. Chem. 1999, 64, 1338–1340.
- 4For the related usage of the ArPb and ArBi species, see
- 4aJ. T. Pinhey, B. A. Rowe, Aust. J. Chem. 1979, 32, 1561–1566;
- 4bD. H. R. Barton, J.-C. Blazejewski, B. Charpiot, D. J. Lester, W. B. Motherwell, M. T. Barros Papoula, J. Chem. Soc. Chem. Commun. 1980, 827–829.
- 5
- 5aM. Ochiai, Y. Kitagawa, M. Toyonari, Arkivoc 2003, 43–48;
- 5bP. O. Norrby, T. B. Petersen, M. Bielawski, B. Olofsson, Chem. Eur. J. 2010, 16, 8251–8254.
- 6
- 6aM. Ochiai, Y. Kitagawa, N. Takayama, Y. Takaoka, M. Shiro, J. Am. Chem. Soc. 1999, 121, 9233–9234;
- 6bV. K. Aggarwal, B. Olofsson, Angew. Chem. Int. Ed. 2005, 44, 5516–5519; Angew. Chem. 2005, 117, 5652–5655;
- 6cA. E. Allen, D. W. C. MacMillan, J. Am. Chem. Soc. 2011, 133, 4260–4263.
- 7
- 7aE. A. Merritt, B. Olofsson, Angew. Chem. Int. Ed. 2009, 48, 9052–9070; Angew. Chem. 2009, 121, 9214–9234;
- 7bJ. Malmgren, S. Santoro, N. Jalalian, F. Himo, B. Olofsson, Chem. Eur. J. 2013, 19, 10334–10342.
- 8For further reading on hypervalent iodine chemistry, see: V. V. Zhdankin, Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, Chichester, 2014.
- 9PhIX2 are also net 2e− oxidants in the α-arylation using ArH:
- 9aY. Kita, H. Tohma, K. Hatanaka, T. Takada, S. Fujita, S. Mitoh, H. Sakurai, S. Oka, J. Am. Chem. Soc. 1994, 116, 3684–3691;
- 9bT. C. Turner, K. Shibayama, D. L. Boger, Org. Lett. 2013, 15, 1100–1103.
- 10E. Faggi, R. M. Sebastián, R. Pleixats, A. Vallribera, A. Shafir, A. Rodríguez-Gimeno, C. Ramírez de Arellano, J. Am. Chem. Soc. 2010, 132, 17980–17982.
- 11The mass balance is made up by the ketone α-oxidation products COH and CO2CF3 and those stemming from oxidative ring-opening.
- 12A. A. Zagulyaeva, M. S. Yusubov, V. V. Zhdankin, J. Org. Chem. 2010, 75, 2119–2122.
- 13For the synthesis of α-cyanoketones: H.-J. Liu, T. W. Ly, C.-L. Tai, J.-D. Wu, J.-K. Liang, J.-C. Guo, N.-W. Tseng, K.-S. Shia, Tetrahedron 2003, 59, 1209–1226.
- 14
- 14aX. L. Huang, N. Maulide, J. Am. Chem. Soc. 2011, 133, 8510–8513;
- 14bX. L. Huang, S. Klimczyk, N. Maulide, Synthesis 2012, 175–183; for a related Au-catalyzed process, see
- 14cA. B. Cuenca, S. Montserrat, K. M. Hossain, G. Mancha, A. Lledós, M. Medio-Simón, G. Ujaque, G. Asensio, Org. Lett. 2009, 11, 4906–4909.
- 15
- 15aE. Malamidou-Xenikaki, S. Spyroudis, Synlett 2008, 2725–2740;
- 15bS. R. Goudreau, D. Marcoux, A. B. Charette, J. Org. Chem. 2009, 74, 470–473;
- 15cK. Gondo, T. Kitamura, Molecules 2012, 17, 6625–6632.
- 16G. F. Koser, A. G. Relenyi, A. N. Kalos, L. Rebrovic, R. H. Wettach, J. Org. Chem. 1982, 47, 2487–2489.
- 17
- 17aM. Ochiai, T. Ito, Y. Takaoka, Y. Masaki, J. Am. Chem. Soc. 1991, 113, 1319–1323;
- 17bM. Ochiai, T. Ito, J. Org. Chem. 1995, 60, 2274–2275;
- 17cH. R. Khatri, J. L. Zhu, Chem. Eur. J. 2012, 18, 12232–12236.
- 18The mechanism was also invoked by Porco et al. to explain the formation of the species C: J. L. Zhu, A. R. Germain, J. A. Porco, Angew. Chem. Int. Ed. 2004, 43, 1239–1243;
Angew. Chem. 2004, 116, 1259–1263.
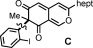
- 19Only the PhI(O2CCF3)2 and the coupling product were detected by NMR during the reaction.
- 20CCDC 1005725 (13), 1005726 (25), 1005727 (31-trans), 1005728 (32-trans) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21For a review on Cu-catalyzed coupling, see: I. P. Beletskaya, A. V. Cheprakov, Coord. Chem. Rev. 2004, 248, 2337–2364; for the Cu-catalyzed N-arylation of amides, see A. Klapars, J. C. Antilla, X. H. Huang, S. L. Buchwald, J. Am. Chem. Soc. 2001, 123, 7727–7729.
- 22For strategies in Gelsemine synthesis: H. Lin, S. J. Danishefsky, Angew. Chem. Int. Ed. 2003, 42, 36–51; Angew. Chem. 2003, 115, 38–53.
- 23In both cases, a competing CO coupling yielded small amounts (5–15 %) of the dihydrobenzofuran 33 (Figure 2, Supporting Information).
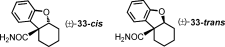
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




