Copper-Doped CdSe/ZnS Quantum Dots: Controllable Photoactivated Copper(I) Cation Storage and Release Vectors for Catalysis†
We are grateful to Dr. Stanislav Strekopytov for the ICP-MS analysis at the Imaging and Analysis Centre, Natural History Museum, London. We also thank the Leeds EPSRC Nanoscience and Nanotechnology Facility for equipment access and the EPSRC for funding N.H..
Abstract
The first photoactivated doped quantum dot vector for metal-ion release has been developed. A facile method for doping copper(I) cations within ZnS quantum dot shells was achieved through the use of metal-dithiocarbamates, with Cu+ ions elucidated by X-ray photoelectron spectroscopy. Photoexcitation of the quantum dots has been shown to release Cu+ ions, which was employed as an effective catalyst for the Huisgen [3+2] cycloaddition reaction. The relationship between the extent of doping, catalytic activity, and the fluorescence quenching was also explored.
The use of quantum dots (QDs) for optical devices,1–3 bio-imaging agents,4, 5 and energy materials6 has been the subject of intensive research, but their photoactivated properties have been largely overlooked as vectors for the controlled release of catalytic metals.7 Herein we demonstrate that Cu-doped CdSe/ZnS QDs act as vectors for the photoinduced release of Cu+ ions for catalysis. The overgrowth of a shell composed of a semiconducting material such as ZnS with a larger band-gap (type-I core–shell system) than the QD core passivates the QD surface and leads to much improved photostability and decreased cytotoxicity.8 The thickness of the shell, as well as the presence of dopants has been shown to dictate QD luminescence properties,9 which we herein link to catalytic potential.
Past studies on Cu doping have focused on doping the QD core, resulting in emission-tunable nanoparticles with enhanced luminescence properties and long-lived photoinduced magnetization.10 When doped into ZnS QD cores, Cu has been shown in the 2+ oxidation state and can act as a source of optically active holes.11 Crooker and co-workers have shown that photoexcitation allows the tuning of the paramagnetic Cu2+ species in the QD core.12 Doping Cu into the QD shells has been largely uninvestigated. This is possibly because of the detrimental effect Cu ions have on the QD luminescence.13 Indeed, QDs have demonstrated high sensitivity towards Cu ions, where the presence of Cu dramatically quenches the QD core photoluminescence.14–16 The oxidation state of Cu in ZnS shells has been discussed for a long time and it has been established that Cu adopts the 1+ oxidation state in ZnS lattices, which was confirmed by our XPS measurements.17
Herein we show that doping small amounts of Cu into the ZnS QD shell partially quenches the QD core luminescence, but makes QDs photoactivated vectors for the release of catalytically active Cu+ ions. We hypothesize that S in the ZnS/CuS shell is slowly photooxidized to SOxy− on irradiation, which causes Cu+ to be released into solution.
To evaluate this hypothesis, reactions which are selectively catalyzed by Cu+ species such as the Huisgen [3+2] cycloaddition developed by Sharpless18 and Meldal19 and co-workers have been employed. To the best of our knowledge, this is the first example of CdSe/ZnS-CuS QDs as Cu+ vectors for heterogeneous catalysis.
Typically, QDs are shelled either by precursor injection,20 epitaxial growth21 or thermal decomposition.22 Herein CdSe core QDs were synthesized using a hot-injection method and shelled according to a modified procedure outlined by Dethlefsen and Døssing.23
The Cu-doping of the ZnS shell used in this study was achieved using a new, one-pot low-temperature thermal decomposition of air-stable precursors in the presence of alkyl surfactants. The Cu dopant and ZnS shelling material were introduced as a stoichiometric mixture of copper(II) diethyldithiocarbamate [(Cu(DEDTC)2)] and zinc(II) diethyldithiocarbamate [(Zn(DEDTC)2)], respectively. Dithiocarbamates are promising single-source precursors for the synthesis of metal sulphide nanoparticles24 and have been used to great effect in ZnS QD shelling.25, 26 Their use as dopant vectors in QD shells is underexplored, and given the wide array of metal dithiocarbamates, spanning the entirety of the periodic metals this shell-doping method has great scope given the myriad of new materials that can be formed.
CdSe cores were shelled by the decomposition of [Zn(DEDTC)2] and [Cu(DEDTC)2] in the following molar ratios; 1:1 (I), 1:3 (II), 1:7 (III), 1:15 (IV), and 0:1 (V; see Table S1 in the Supporting Information). The total molar quantity of shell precursor was identical to that required for the CdSe/ZnS, equivalent to six monolayers.23
The presence of Cu in the QDs was shown by X-ray photoelectron spectroscopy (XPS). The molar ratios of Cu to Zn used in the QD shelling relates directly to the intensity of the Cu and Zn signals in the XPS spectra (Section S1). The Cu2p3/2 and Cu2p1/2 peaks for all samples are reported as 933.18 and 952.88 eV, respectively, which is indicative of the presence of Cu+ ions.13
Energy-dispersive X-ray (EDX) spectroscopy (Figure 1) confirmed the presence of all of the elements in the structure (Cd, Se, Zn, S, and Cu), versus CdSe/ZnS QDs. Quantitative EDX analysis showed the amount of Cu loading on the QDs, all of which were low in comparison to the stoichiometric amounts of shelling reagents used (Table S1). XPS analysis gave higher Cu-to-Zn ratios than EDX, thus suggesting the majority of Cu sites are located on the QD surface. We can also infer that the CdSe cores have a preferential affinity for ZnS over CuS shelling because of the higher amount of Zn in the system. Further characterization of I–V by transmission electron microscopy (TEM) showed all samples to be monodisperse with an average diameter of 3.7 to 4.1 nm (Section S2). High-resolution TEM (HRTEM) measurements showed highly crystalline structures with d-spacings corresponding to the 〈111〉 crystal plane of CdSe (see insets in Figure 1).
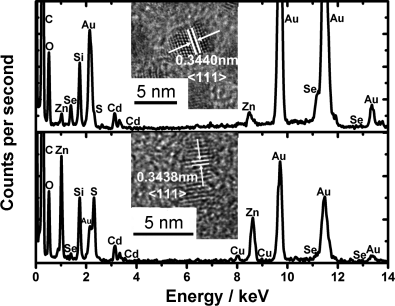
EDX spectra and HRTEM images of CdSe/ZnS and CdSe/ZnS/CuS nanocrystals.
Figure 2 A shows the absorption and photoluminescence (PL) spectra of samples I–V. When Cu is introduced to the ZnS shell, a blue shift in luminescence and a decrease in quantum yield were observed. This is in stark contrast to a pure ZnS shell, where a significant increase in quantum yield and a red-shift in luminescence is observed. In the case of sample I, the PL was completely quenched. Furthermore, Cu-doped samples did show an increased near-infrared PL band at approximately 1.8 eV (660 nm) (Figure 2 B), which had a significantly longer luminescence life-time compared with the band-edge luminescence (Figure 2 D). If we assume that Cu+ ions have been doped into the ZnS shell (which is reasonable given the low levels of Cu found in the particles experimentally), Figure 2 C represents the energy levels in the QDs.11, 27–29 Cu-doped ZnS shows luminescence in the blue/green region of the spectrum because of S and Zn vacancies and a level introduced by the Cu+ ions.28 This luminescence is not visible in our spectra and is most likely quenched by the CdSe core or not intense enough to be detected. The near-infrared luminescence at about 1.8 eV (660 nm) is well-known in CdSe nanocrystals and can be assigned to Se or Se/Cd (di)vacancies probably located at the boundary between core and shell.27, 30

A) UV/Vis and fluorescence spectra of samples (I–V). B) Fluorescence spectra of samples II and V clearly showing a second, broad emission band centered at 660 nm for sample II. C) Fluorescence decay measurements at 540 and 660 nm, illustrating the long-lived Cu-induced feature at 660 nm. D) Proposed fluorescence energy level diagram.
The blue-shift in the core luminescence may be due to a “shrinking” of the CdSe core or changes in its dielectric environment. Given the low levels of Cu doping, the Cu in the “shelling” reagents partially exchanged against Cd. This is plausible bearing in mind work on Cu activation of chalcogenides and cation exchange.13, 31 The “shrinking” theory is further supported by the increase in the near-infrared photoluminescence, which indicates that the number of defect states in the core has been increased—especially at the interface between core and shell.
As Cu in the ZnS shell has Cu+ character, excitation of the QDs is likely to oxidize the Cu dopants releasing Cu+ ions into solution. The mechanism of release likely involves photooxidation of the doped ZnS shell under release of Cu, Zn, and SOxy− ions. The proposed mechanism was supported by the detection of Cu using inductively coupled plasma-mass spectroscopy (ICP-MS). A QD suspension of I in hexane was irradiated with 254 nm overnight, with a counterpart QD suspension kept in the dark. The QDs were precipitated with ethanol, centrifuged, and the supernatant evaporated for nitric acid digestion and analysis. The irradiated sample leached about 14 times as much Cu compared to the dark sample, which supports the hypothesis of photooxidation promoted Cu+ release (Section S3.5). The amount of copper leached is small (1033 ng ±10 % from a total QD mass of 16.2 mg).
A practical way of using the as-generated Cu+ species is the Huisgen [3+2] cylcloaddition which is known to be catalyzed by Cu+ sites more readily than Cu2+. The literature precedent is to start with a Cu2+ salt and generate in situ Cu+ for catalysis.32 Commonly used in situ reducing agents such as sodium ascorbate derivatives have drawbacks in bioconjugation applications because of separate reactions with protein side-chains.33, 34 The groups of Bowman and Yagci have independently shown that separate UV and visible-light activators can be used to reduce Cu2+ to Cu+ in a two component system using a radical reduction mechanism, however the selectivity of the radical photoreducing agent is unclear.35–37 As such, a system that can release Cu+ in a controlled manner without the need for a reducing agent or radical initiator would be highly advantageous in bioconjugation.
Sample I, containing the highest amount of Cu doped in the ZnS shell (Section S7), was tested first. 1H NMR spectroscopy was used to monitor the azide versus triazole ratio (Figure 3, Ha and Hb, respectively). A concentration with 5 mol % of Cu in hexane (1 mL) was shown to have reached >99 % yield within 2 h. The insolubility of the triazole in hexane allows separation from the QDs by filtration. Analyzing aliquots at timed intervals by 1H NMR spectroscopy shows 1, 2, 8, 35, and 100 % of triazole formed at 15, 30, 60, 90, and 120 minutes, respectively. The slow initial product formation suggests there is an activation period after irradiation of 60 minutes. Presumably, this initial inactivity is due to the QDs being kept in the dark prior to use. The activation period could be postulated to be the time required for the release of Cu+ ions and is therefore rate-limiting.
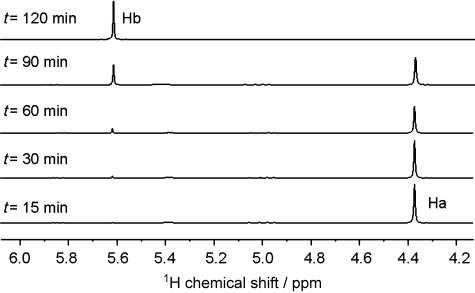
1H NMR spectroscopy of the azide–alkyne cycloaddition catalyzed by I (5 mol % Cu).
Performing the same reaction in the dark gave a 22 % yield. Therefore, we postulate that on irradiation, the QDs photooxidize with S being oxidized to SOxy−. This causes the Cu to form a complex with the azide, which acts as a sacrificial electron acceptor and subsequently, the click reaction occurs.
Reducing the QD loading of I to 0.32 mol % (5 wt % of I) gives >99 % yield after 8 h (Table 1). Repeating three cycles using the same catalyst has no detrimental effect on yield showing the QD core is able to release catalytic Cu+ over a prolonged period. On comparison, the lower level of doping in II reduces the mole percentage (mol %) of Cu to 0.16 % versus the same 5 wt % of QDs in the reaction; this reduction has no detrimental effect affording a yield greater than 99 % after 8 h with a turnover number (TON) of 625. Reactions involving QDs III and IV (5 wt %) show a sharp decline in yield and this can be correlated to the low proportion of Cu doping in these samples. The assumption that the catalysis is taking place on a Cu center is supported by sample V whereby no reaction takes place without the presence of Cu sites. The reaction also proceeds with visible light activation with a yield greater than 99 % after 3 h using I (5 mol % Cu).
|
Catalyst |
QD [wt %] |
Cu [mol %] |
T [h] |
Yield+hν[d] [%] |
TON |
TOF [h−1][e] |
Yield−hν [%] |
|---|---|---|---|---|---|---|---|
|
none |
0 |
0.00 |
8 |
<0.1 |
0 |
0 |
0 |
|
I |
77.2 |
5.00 |
2 |
>99 |
20 |
10 |
22 |
|
I |
5 |
0.32 |
8 |
>99[a], >99[b], >99[c] |
309 |
39 |
11 |
|
II |
5 |
0.16 |
8 |
>99 |
625 |
78 |
5 |
|
III |
5 |
0.12 |
8 |
7.50 |
61 |
8 |
<0.1 |
|
IV |
5 |
0.04 |
8 |
1.30 |
32 |
4 |
<0.1 |
|
V |
5 |
0.00 |
8 |
<0.1 |
0 |
0 |
<0.1 |
- [a], [b], [c] Repeats using the same catalyst. [d] hν used; 257 nm 980 μW cm−1. [e] TOF=turnover frequency.
Experimental Section
Synthesis of [Cu(DEDTC)2]: Cu(S2CN(CH2CH3)2)2 was synthesized using a modified procedure outlined by others.38
Synthesis of CdSe QD cores: Luminescent CdSe cores were synthesized following an adapted hot-injection method as described by Roullier et al. with modifications.20
Shelling of CdSe QDs with ZnS: A shell of ZnS was synthesized by thermal decomposition of [Zn(DEDTC)2] according to a procedure outlined by Dethlefsen and Døssing.23
Synthesis of CdSe/ZnS-CuS core–shell quantum dots: Cu was doped into the ZnS shell using a modified procedure outlined by Dethlefsen and Døssing.23 A mixture of [Zn(DEDTC)2] and [Cu(DEDTC)2] composed of different molar ratios was used as the Zn, Cu, and S precursors, respectively (Table S1).




