The Critical Effect of the Countercation in the Direct Cupration of Fluoroform with [Cu(OR)2]−†
Andrey I. Konovalov
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorDr. Jordi Benet-Buchholz
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorDr. Eddy Martin
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Vladimir V. Grushin
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)Search for more papers by this authorAndrey I. Konovalov
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorDr. Jordi Benet-Buchholz
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorDr. Eddy Martin
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Vladimir V. Grushin
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)Search for more papers by this authorWe thank Prof. S. A. Macgregor and S. Kazandjian for discussions and preliminary computational studies. The ICIQ Foundation and The Spanish Government (Grant CTQ2011-25418) are acknowledged for support of this work.
Graphical Abstract
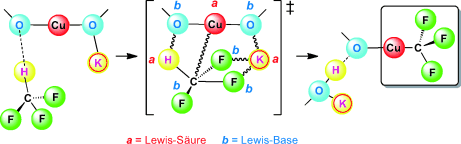
Zuschauer oder Hauptdarsteller? Das Alkalimetall-Gegenion (K+) spielt eine bemerkenswerte Schlüsselrolle in der kürzlich entdeckten Cuprierung von Fluoroform mit Dialkoxycuprat. Insgesamt acht synergistisch wechselwirkende Lewis-Säure- und Lewis-Base-Zentren ordnen sich zu einem stabilen Übergangszustand, wodurch der niederenergetische Reaktionsweg dieser Umwandlung bedingt wird.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201306272_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Han, Y. Li, H. Tang, H. Liu, J. Fluorine Chem. 2012, 140, 7.
- 2O. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475.
- 3For selected recent monographs, see:
- 3aP. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 3bK. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, UK, 2006;
10.1002/9780470988589 Google Scholar
- 3cI. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, UK, 2009;
10.1002/9781444312096 Google Scholar
- 3dV. A. Petrov, Fluorinated Heterocyclic Compounds. Synthesis Chemistry and Applications, Wiley, Hoboken, 2009.
10.1002/9780470528952 Google Scholar
- 4For selected recent reviews of trifluoromethylation methods, see:
- 4aM. Schlosser, Angew. Chem. 2006, 118, 5558;
10.1002/ange.200600449 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5432;
- 4bD. Cahard, J.-A. Ma, J. Fluorine Chem. 2007, 128, 975;
- 4cK. Uneyama, T. Katagiri, H. Amii, Acc. Chem. Res. 2008, 41, 817;
- 4dJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1;
- 4eN. Shibata, A. Matsnev, D. Cahard, Beilstein J. Org. Chem. 2010, 6, 65;
- 4fK. Sato, A. Tarui, M. Omote, A. Ando, I. Kumadaki, Synthesis 2010, 1865;
- 4gS. Roy, B. T. Gregg, G. W. Gribble, V.-D. Le, S. Roy, Tetrahedron 2011, 67, 2161;
- 4hF.-L. Qing, F. Zheng, Synlett 2011, 1052;
- 4iJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2011, 111, 455;
- 4jY. Macé, E. Magnier, Eur. J. Org. Chem. 2012, 2479.
- 5E. A. Symons, M. J. Clermont, J. Am. Chem. Soc. 1981, 103, 3127.
- 6
- 6aT. Shono, M. Ishifune, T. Okada, S. Kashimura, J. Org. Chem. 1991, 56, 2;
- 6bN. Roques, J. Russell, PCT Int. Appl. WO 97/19038, 1997;
- 6cN. Roques, J. Russell, U.S. Patent 6355849, 2002;
- 6dJ. Russell, N. Roques, Tetrahedron 1998, 54, 13771;
- 6eR. Barhdadi, M. Troupel, M. Perichon, Chem. Commun. 1998, 1251;
- 6fB. Folléas, I. Marek, J.-F. Normant, L. Saint-Jalmes, Tetrahedron Lett. 1998, 39, 2973;
- 6gB. Folléas, I. Marek, J.-F. Normant, L. Saint-Jalmes, Tetrahedron 2000, 56, 275;
- 6hT. Billard, S. Bruns, B. R. Langlois, Org. Lett. 2000, 2, 2101;
- 6iS. Large, N. Roques, B. R. Langlois, J. Org. Chem. 2000, 65, 8848;
- 6jB. R. Langlois, T. Billard, ACS Symp. Ser. 2005, 911, 57;
- 6kG. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324;
- 6lH. Kawai, Z. Yuan, E. Tokunaga, N. Shibata, Org. Biomol. Chem. 2013, 11, 1446.
- 7I. Popov, S. Lindeman, O. Daugulis, J. Am. Chem. Soc. 2011, 133, 9286.
- 8A. Zanardi, M. A. Novikov, E. Martin, J. Benet-Buchholz, V. V. Grushin, J. Am. Chem. Soc. 2011, 133, 20901.
- 9P. Novák, A. Lishchynskyi, V. V. Grushin, Angew. Chem. 2012, 124, 7887; Angew. Chem. Int. Ed. 2012, 51, 7767.
- 10P. Novák, A. Lishchynskyi, V. V. Grushin, J. Am. Chem. Soc. 2012, 134, 16167.
- 11CCDC 950993 (2), 950994 (3), 950995 (4), and 950996 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12Reported CuCF3 bond lengths: [(TMS-IPr)Cu(CF3)] (1.967(6) Å),[13a] [(SIiPr)Cu(CF3)] (2.022(4) Å),[13a] [(SIMes)2Cu][(CF3)2Cu] (1.970 (6) Å),[13b] [(phen)Cu(PPh3)(CF3)] (1.985(1) Å),[13c] [(bathophen)Cu(CF3)] (1.907(9) Å),[13d] [(Ph3P)3Cu(CF3)] (2.018(7), 2.025(7), and 2.031(10) Å),[13e] and [Cu4(CF3)2(C(OBu-t)2)2(μ3-OBu-t)2] (1.8908(16) Å).[8]
- 13aG. G. Dubinina, H. Furutachi, D. A. Vicic, J. Am. Chem. Soc. 2008, 130, 8600;
- 13bG. G. Dubinina, J. Ogikubo, D. A. Vicic, Organometallics 2008, 27, 6233;
- 13cH. Morimoto, T. Tsubogo, N. D. Litvinas, J. F. Hartwig, Angew. Chem. 2011, 123, 3877;
10.1002/ange.201100633 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3793;
- 13dZ. Weng, R. Lee, W. Jia, Y. Yuan, W. Wang, X. Feng, K.-W. Huang, Organometallics 2011, 30, 3229;
- 13eO. A. Tomashenko, E. C. Escudero-Adan, M. Martinez Belmonte, V. V. Grushin, Angew. Chem. 2011, 123, 7797;
10.1002/ange.201101577 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7655.
- 14
- 14aI. Takeda, Bull. Chem. Soc. Jpn. 1981, 54, 3133;
- 14bK. Ozutsumi, K. Ohtsu, T. Kawashima, J. Chem. Soc. Faraday Trans. 1994, 90, 127.
- 15J. Gutknecht, H. Schneider, J. Stroka, Inorg. Chem. 1978, 17, 3326.
- 16The reported[14, 15] log KS values were obtained for KClO4. As [(tBuO)2Cu]− is a stronger coordinating anion than ClO4−, the calculations might somewhat underestimate the actual concentrations of 18-crown-6- and crypt-222-free K+. The overall trend in [K+] and its correlation with the reactivity toward CHF3, however, remain unaffected.
- 17D. Balcells, E. Clot, O. Eisenstein, Chem. Rev. 2010, 110, 749.
- 18See, for example:
- 18aC. J. Creswell, A. L. Allred, J. Am. Chem. Soc. 1963, 85, 1723;
- 18bS. Andreades, J. Am. Chem. Soc. 1964, 86, 2003;
- 18cI. Alkorta, S. Maluendes, J. Phys. Chem. 1995, 99, 6457;
- 18dM. L. Chabinyc, J. I. Brauman, J. Am. Chem. Soc. 1998, 120, 10863;
- 18eA. Mukhopadhyay, P. Pandey, T. Chakraborty, J. Phys. Chem. A 2010, 114, 5026;
- 18fS. J. Grabowski, J. Phys. Chem. A 2011, 115, 12789;
- 18gP. Ramasami, T. A. Ford, J. Mol. Struct. 2012, 1023, 163.
- 19S. Grimme, J. Comput. Chem. 2006, 27, 1787.
- 20See the Supporting Information for details.
- 21Replacing the MeO ligands in the small model with tBuO raised the barrier by 2.5 kcal mol−1.
- 22Recomputing this transition state at the DFT/B3LYP and the DFT/PBE levels produced higher activation barriers of 25.7 and 23.7 kcal mol−1, respectively, thus indicating that the contribution from the London dispersion forces is not negligible.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.