Asymmetric Copolymerization of CO2 with meso-Epoxides Mediated by Dinuclear Cobalt(III) Complexes: Unprecedented Enantioselectivity and Activity†
Ye Liu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorWei-Min Ren
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorJie Liu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Bing Lu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)Search for more papers by this authorYe Liu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorWei-Min Ren
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorJie Liu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Bing Lu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024 (P. R. China)Search for more papers by this authorGratitude is expressed to the National Natural Science Foundation of China (NSFC, Grant 21134002, 21104007), and National Basic Research Program of China (973 Program: 2009CB825300). X.-B. Lu gratefully acknowledges the Chang Jiang Scholars Program (T2011056) from the Ministry of Education of the People’s Republic of China. We are also grateful to Prof. Cheng He and Dr. Haiyan An for their kind assistance in X-ray crystallographic analysis.
Graphical Abstract
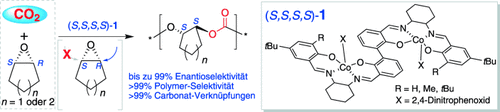
Isotaktisch: In der asymmetrischen Copolymerisation von CO2 mit meso-Epoxiden (darunter das wenig reaktive Cyclopentenoxid), die von dem zweikernigen CoIII-Komplex (S,S,S,S)-1 unter milden Bedingungen katalysiert wurde, wurde beispiellose Enantioselektivität und katalytische Aktivität beobachtet. Die Copolymere enthalten mehr als 99 % Carbonateinheiten und sind perfekt isotaktisch.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201305154_sm_miscellaneous_information.pdf931.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. M. Hodgson, A. R. Gibbs, G. P. Lee, Tetrahedron 1996, 52, 14361–14384;
- 1bE. N. Jacobsen, M. H. Wu in Comprehensive Asymmetric Catalysis, Vols. I–III (Eds.: ), Springer, Berlin, 1999, pp. 1309–1312;
10.1007/978-3-642-58571-5_13 Google Scholar
- 1cM. C. Willis, J. Chem. Soc. Perkin Trans. 1 1999, 1765–1784.
- 2K. Nozaki, K. Nakano, T. Hiyama, J. Am. Chem. Soc. 1999, 121, 11008–11009.
- 3M. Cheng, N. A. Darling, E. B. Lobkovsky, G. W. Coates, Chem. Commun. 2000, 2007–2008.
- 4
- 4aK. Nakano, K. Nozaki, T. Hiyama, J. Am. Chem. Soc. 2003, 125, 5501–5510;
- 4bK. Nakano, T. Hiyama, K. Nozaki, Chem. Commun. 2005, 1871–1873;
- 4cS. Abbina, G. D. Du, Organometallics 2012, 31, 7394–7403.
- 5Y. Xiao, Z. Wang, K. Ding, Chem. Eur. J. 2005, 11, 3668–3678.
- 6K. Nishioka, H. Goto, H. Sugimoto, Macromolecules 2012, 45, 8172–8192.
- 7
- 7aG. P. Wu, W. M. Ren, Y. Luo, B. Li, W. Z. Zhang, X. B. Lu, J. Am. Chem. Soc. 2012, 134, 5682–5688;
- 7bG. P. Wu, S. D. Jiang, X. B. Lu, W. M. Ren, S. K. Yan, Chin. J. Polym. Sci. 2012, 30, 487–492.
- 8
- 8aE. N. Jacobsen, Acc. Chem. Res. 2000, 33, 421–431;
- 8bL. E. Martinez, J. L. Leighton, D. H. Carsten, E. N. Jacobsen, J. Am. Chem. Soc. 1995, 117, 5897–5898;
- 8cR. G. Konsler, J. Karl, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 10780–10781.
- 9
- 9aK. Nakano, S. Hashimotoa, K. Nozaki, Chem. Sci. 2010, 1, 369–373;
- 9bS. I. Vagin, R. Reichardt, S. Klaus, B. Rieger, J. Am. Chem. Soc. 2010, 132, 14367–14369;
- 9cS. Klaus, S. I. Vagin, M. W. Lehenmeier, P. Deglmann, A. K. Brym, B. Rieger, Macromolecules 2011, 44, 9508–9516;
- 9dS. Klaus, M. W. Lehenmeier, C. E. Anderson, B. Rieger, Coord. Chem. Rev. 2011, 255, 1460–1479.
- 10aM. R. Kember, P. D. Knight, P. T. R. Reung, C. K. Williams, Angew. Chem. 2009, 121, 949–951;
10.1002/ange.200803896 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 931–933;
- 10bM. R. Kember, A. J. P. White, C. K. Williams, Macromolecules 2010, 43, 2291–2298;
- 10cF. Jutz, A. Buchard, M. R. Kember, S. B. Fredriksen, C. K. Williams, J. Am. Chem. Soc. 2011, 133, 17395–17405;
- 10dM. R. Kember, C. K. Williams, J. Am. Chem. Soc. 2012, 134, 15676–15679;
- 10eM. R. Kember, F. Jutz, A. Buchard, A. J. P. White, C. K. Williams, Chem. Sci. 2012, 3, 1245–1255.
- 11
- 11aD. J. Darensbourg, J. R. Wildeson, J. C. Yarbrough, J. H. Reibenspies, J. Am. Chem. Soc. 2000, 122, 12487–12496;
- 11bB. Y. Liu, X. J. Zhao, X. H. Wang, F. S. Wang, J. Polym. Sci. Part A 2001, 39, 2751–2754;
- 11cD. R. Moore, M. Cheng, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2003, 125, 11911–11924;
- 11dB. Y. Lee, H. Y. Kwon, S. J. Na, S. Han, H. Yun, H. Lee, Y. W. Park, J. Am. Chem. Soc. 2005, 127, 3031–3037;
- 11eR. J. Wei, X. H. Zhang, B. Y. Du, X. K. Sun, Z. Q. Fan, G. R. Qi, Macromolecules 2013, 46, 3693–3697.
- 12
- 12aX. B. Lu, W. M. Ren, G. P. Wu, Acc. Chem. Res. 2012, 45, 1721–1735;
- 12bW. M. Ren, Z. W. Liu, Y. Q. Wen, R. Zhang, X. B. Lu, J. Am. Chem. Soc. 2009, 131, 11509–11518;
- 12cX. B. Lu, L. Shi, Y. M. Wang, R. Zhang, Y. J. Zhang, X. J. Peng, Z. C. Zhang, B. Li, J. Am. Chem. Soc. 2006, 128, 1664–1674;
- 12dX. B. Lu, Y. Wang, Angew. Chem. 2004, 116, 3658–3661; Angew. Chem. Int. Ed. 2004, 43, 3574–3577.
- 13aC. T. Cohen, T. Chu, G. W. Coates, J. Am. Chem. Soc. 2005, 127, 10869–10878;
- 13bR. L. Paddock, S. T. Nguyen, Macromolecules 2005, 38, 6251–6253;
- 13cC. T. Cohen, G. W. Coates, J. Polym. Sci. Part A 2006, 44, 5182–5191;
- 13dX. B. Lu, D. J. Darensbourg, Chem. Soc. Rev. 2012, 41, 1462–1484.
- 14
- 14aK. Nakano, T. Kamada, K. Nozaki, Angew. Chem. 2006, 118, 7432–7435; Angew. Chem. Int. Ed. 2006, 45, 7274–7277;
- 14bE. K. Noh, S. J. Na, S. Sujith, S. W. Kim, B. Y. Lee, J. Am. Chem. Soc. 2007, 129, 8082–8083;
- 14cS. Sujith, K. K. Min, J. E. Seong, S. J. Na, B. Y. Lee, Angew. Chem. 2008, 120, 7416–7419;
10.1002/ange.200801852 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7306–7309;
- 14dK. Nakano, S. Hashimoto, M. Nakamura, T. Kamada, K. Nozaki, Angew. Chem. 2011, 123, 4970–4973; Angew. Chem. Int. Ed. 2011, 50, 4868–4871.
- 15Usually, the addition of a nucleophilic cocatalyst greatly increases the catalytic activity of both mono- and dinuclear CoIII complexes for CO2/epoxide copolymerization (See Refs. [9a, 12, 13]). However, in comparison with 1 a, 1 b, or 1 c alone as catalyst, the presence of cocatalyst PPNX resulted in a slight decreases in catalytic activity for CO2/CPO copolymerization, but great increases in activity for CO2/CHO copolymerization. This indicates the different propensity of the two meso-epoxides during the copolymerization with CO2.
- 16
- 16aW. Hirahata, R. M. Thomas, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2008, 130, 17658–17659;
- 16bR. M. Thomas, P. C. B. Widger, S. M. Ahmed, R. C. Jeske, W. Hirahata, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2010, 132, 16520–16525.
- 17Since the X-ray structure of the mononuclear SalenAlIIICl complex is similar with that of SalenCoIIICl bearing a cyclohexane diamine backone {see:
- 17aD. J. Darensbourg, D. R. Billodeaux, Inorg. Chem. 2005, 44, 1433–1742;
- 17bC. T. Cohen, C. M. Thomas, K. L. Peretti, E. B. Lobkovsky, G. W. Coates, Dalton Trans. 2006, 237–249}, we believed that the structure of dinuclear SalenAlIIICl can reflect the structural information of the corresponding dinuclear SalenCoIII complex.
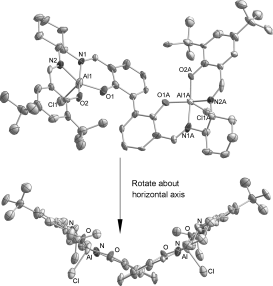 Molecular structure of dinuclear SalenAlIIICl (hydrogen atoms and uncoordinated solvent omitted for clarity; carbon atoms are unlabeled). Thermal ellipsoids are at the 30 % probability level. The complex displays an Al–Al distance of 7.89 Å and an exo phenyl–phenyl dihedral angle of 45.1°.
Molecular structure of dinuclear SalenAlIIICl (hydrogen atoms and uncoordinated solvent omitted for clarity; carbon atoms are unlabeled). Thermal ellipsoids are at the 30 % probability level. The complex displays an Al–Al distance of 7.89 Å and an exo phenyl–phenyl dihedral angle of 45.1°.
- 18Notably, the use of other nucleophilic cocatalysts, such as simple nBu4NCl, could significantly improve the activity of the dinuclear CoIII catalyst. However, the copolymerization is very sensitive to water, so the avoidance of water or the decrease to a great extent in the reaction system is necessary for obtaining a high reaction rate and copolymers with highly molecular weight.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.