Peptide-Catalyzed Regio- and Enantioselective Reduction of α,β,γ,δ-Unsaturated Aldehydes†
Dr. Kengo Akagawa
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Search for more papers by this authorJun Sen
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kazuaki Kudo
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)Search for more papers by this authorDr. Kengo Akagawa
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Search for more papers by this authorJun Sen
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kazuaki Kudo
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505 (Japan)Search for more papers by this authorThis work was supported by JSPS KAKENHI Grant Number 23750171 (to K.A.) and 23550116 (to K.K.), and by MEXT KAKENHI Grant number 24105506 (to K.K.).
Graphical Abstract
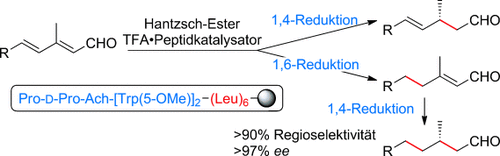
Ein Peptidkatalysator an fester Phase (abgebildet im Kasten) wurde in der Titelreaktion verwendet. Der Katalysator umgeht die inhärente Regioselektivität der Reaktion, sodass die 1,6- gegenüber der 1,4-Reduktion bevorzugt wird. Ausgehend von einem Gemisch geometrischer Isomere der eingesetzten Aldehyde wurde eine hohe Stereokonvergenz erzielt. Ach=1-Amino-1-cyclohexancarbonsäure.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201305004_sm_miscellaneous_information.pdf13.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A review: J. Mahatthananchai, A. M. Dumas, J. W. Bode, Angew. Chem. 2012, 124, 11114–11152;
10.1002/ange.201201787 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10954–10990.
- 2Selected examples:
- 2aM. J. Martinelli, R. Vaidyanathan, J. M. Pawlak, N. K. Nayyar, U. P. Dhokte, C. W. Doecke, L. M. H. Zollars, E. D. Moher, V. Van Khau, B. Košmrlj, J. Am. Chem. Soc. 2002, 124, 3578;
- 2bT. Fujihara, K. Semba, J. Terao, Y. Tsuji, Angew. Chem. 2010, 122, 1514–1518;
10.1002/ange.200906348 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1472–1476;
- 2cA. D. Worthy, X. Sun, K. L. Tan, J. Am. Chem. Soc. 2012, 134, 7321–7324;
- 2dA. Di Giuseppe, R. Castarlenas, J. J. Pérez-Torrente, M. Crucianelli, V. Polo, R. Sancho, F. J. Lahoz, L. A. Oro, J. Am. Chem. Soc. 2012, 134, 8171–8183;
- 2eJ. Lv, L. Zhang, Y. Zhou, Z. Nie, S. Luo, J.-P. Cheng, Angew. Chem. 2011, 123, 6740–6744; Angew. Chem. Int. Ed. 2011, 50, 6610–6614;
- 2fL. Gremaud, A. Alexakis, Angew. Chem. 2012, 124, 818–821;
10.1002/ange.201107324 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 794–797;
- 2gC. Zheng, D. Wang, S. S. Stahl, J. Am. Chem. Soc. 2012, 134, 16496–16499;
- 2hJ. L. Farmer, H. N. Hunter, M. G. Organ, J. Am. Chem. Soc. 2012, 134, 17470–17473;
- 2iD. Lee, M. S. Taylor, J. Am. Chem. Soc. 2011, 133, 3724–3727;
- 2jW. Muramatsu, Y. Takemoto, J. Org. Chem. 2013, 78, 2336–2345.
- 3
- 3aC. A. Lewis, S. J. Miller, Angew. Chem. 2006, 118, 5744–5747; Angew. Chem. Int. Ed. 2006, 45, 5616–5619;
- 3bP. A. Jordan, S. J. Miller, Angew. Chem. 2012, 124, 2961–2965; Angew. Chem. Int. Ed. 2012, 51, 2907–2911;
- 3cP. A. Lichtor, S. J. Miller, Nat. Chem. 2012, 4, 990–995.
- 4
- 4aT. Kawabata, T. Furuta, Chem. Lett. 2009, 38, 640–647;
- 4bK. Yoshida, T. Shigeta, T. Furuta, T. Kawabata, Chem. Commun. 2012, 48, 6981–6983.
- 5Reviews:
- 5aN. Krause, S. Thorand, Inorg. Chim. Acta 1999, 296, 1–11;
- 5bA. G. Csákÿ, G. de La Herrán, M. C. Murcia, Chem. Soc. Rev. 2010, 39, 4080–4102.
- 6Selected examples:
- 6aJ. Munch-Petersen, C. Bretting, P. M. Jørgensen, S. Refn, V. K. Andersen, A. Jart, Acta Chem. Scand. 1961, 15, 277–292;
- 6bY. Yamamoto, K. Maruyama, J. Am. Chem. Soc. 1978, 100, 3240–3241;
- 6cT. den Hartog, S. R. Harutyunyan, D. Font, A. J. Minnaard, B. L. Feringa, Angew. Chem. 2008, 120, 404–407;
10.1002/ange.200703702 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 398–401;
- 6dS. Okada, K. Arayama, R. Murayama, T. Ishizuka, K. Hara, N. Hirone, T. Hata, H. Urabe, Angew. Chem. 2008, 120, 6966–6970; Angew. Chem. Int. Ed. 2008, 47, 6860–6864;
- 6eH. Hénon, M. Mauduit, A. Alexakis, Angew. Chem. 2008, 120, 9262–9264;
10.1002/ange.200803735 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9122–9124;
- 6fT. Nishimura, Y. Yasuhara, T. Sawano, T. Hayashi, J. Am. Chem. Soc. 2010, 132, 7872–7873;
- 6gT. Sawano, A. Ashouri, T. Nishimura, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 18936–18939.
- 7
- 7aL. Bernardi, J. López-Cantarero, B. Niess, K. A. Jørgensen, J. Am. Chem. Soc. 2007, 129, 5772–5778;
- 7bD. Uraguchi, K. Yoshida, Y. Ueki, T. Ooi, J. Am. Chem. Soc. 2012, 134, 19370–19373.
- 8Other selected examples of organocatalytic regioselective reactions:
- 8aS. Belot, A. Quintard, N. Krause, A. Alexakis, Adv. Synth. Catal. 2010, 352, 667–695;
- 8bD. Enders, C. Wang, A. Greb, Adv. Synth. Catal. 2010, 352, 987–992;
- 8cP. Chauhan, S. S. Chimni, Adv. Synth. Catal. 2011, 353, 3203–3212;
- 8dJ. J. Murphy, A. Quintard, P. McArdle, A. Alexakis, J. C. Stephens, Angew. Chem. 2011, 123, 5201–5204;
10.1002/ange.201100804 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5095–5098;
- 8eJ. Wang, J. Chen, C. W. Kee, C.-H. Tan, Angew. Chem. 2012, 124, 2432–2436; Angew. Chem. Int. Ed. 2012, 51, 2382–2386;
- 8fŁ. Albrecht, G. Dickmeiss, F. C. Acosta, C. Rodríguez-Escrich, R. L. Davis, K. A. Jørgensen, J. Am. Chem. Soc. 2012, 134, 2543–2546;
- 8gF. Zhong, J. Luo, G.-Y. Chen, X. Dou, Y. Lu, J. Am. Chem. Soc. 2012, 134, 10222–10227;
- 8hK. S. Halskov, T. K. Johansen, R. L. Davis, M. Steurer, F. Jensen, K. A. Jørgensen, J. Am. Chem. Soc. 2012, 134, 12943–12946.
- 9Reviews:
- 9aA. Erkkilä, I. Majander, P. M. Pihko, Chem. Rev. 2007, 107, 5416–5470;
- 9bS. Mukherjee, J. W. Yang, S. Hoffman, B. List, Chem. Rev. 2007, 107, 5471–5569.
- 10Recent selected examples:
- 10aW. Zhang, W. L. Tang, Z. Wang, Z. Li, Adv. Synth. Catal. 2010, 352, 3380–3390;
- 10bR. C. Simon, B. Grischek, F. Zepeck, A. Steinreiber, F. Belaj, W. Kroutil, Angew. Chem. 2012, 124, 6817–6820;
10.1002/ange.201202375 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6713–6716;
- 10cA. Bariotaki, D. Kalaitzakis, I. Smonou, Org. Lett. 2012, 14, 1792–1795.
- 11Reviews of peptide catalysts:
- 11aE. A. C. Davie, S. M. Mennen, Y. Xu, S. J. Miller, Chem. Rev. 2007, 107, 5759–5812;
- 11bH. Wennemers, Chem. Commun. 2011, 47, 12036–12041.
- 12
- 12aK. Akagawa, H. Akabane, S. Sakamoto, K. Kudo, Org. Lett. 2008, 10, 2035–2037;
- 12bK. Akagawa, H. Akabane, S. Sakamoto, K. Kudo, Tetrahedron: Asymmetry 2009, 20, 461–466.
- 13
- 13aJ. W. Yang, M. T. H. Fonseca, N. Vignola, B. List, Angew. Chem. 2005, 117, 110–112; Angew. Chem. Int. Ed. 2005, 44, 108–110;
- 13bS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 32–33.
- 14Reviews of organocatalytic reductions with Hantzsch esters:
- 14aS. G. Ouellet, A. M. Walji, D. W. C. MacMillan, Acc. Chem. Res. 2007, 40, 1327–1339;
- 14bS.-L. You, Chem. Asian J. 2007, 2, 820–827;
- 14cM. Rueping, E. Sugiono, F. R. Schoepke, Synlett 2010, 852–865.
- 15
- 15aT. S. Haque, J. C. Little, S. H. Gellman, J. Am. Chem. Soc. 1996, 118, 6975–6985;
- 15bJ. T. Blank, S. J. Miller, Biopolymers 2006, 84, 38–47.
- 16G. B. Fields, R. L. Noble, Int. J. Pept. Protein Res. 1990, 35, 161–214.
- 17Detaching a peptide from the resin leads to a decrease in both reactivity and stereoselectivity, probably because of low solubility, see: K. Akagawa, R. Suzuki, K. Kudo, Adv. Synth. Catal. 2012, 354, 1280–1286.
- 18Y. Hayashi, D. Okamura, S. Umemiya, T. Uchimaru, ChemCatChem 2012, 4, 959–962.
- 19
- 19aX. Tian, Y. Liu, P. Melchiorre, Angew. Chem. 2012, 124, 6545–6548; Angew. Chem. Int. Ed. 2012, 51, 6439–6442;
- 19bX. Tian, P. Melchiorre, Angew. Chem. 2013, 125, 5468–5471; Angew. Chem. Int. Ed. 2013, 52, 5360–5363.
- 20
- 20aL. Dell’Amico, Ł. Albrecht, T. Naicker, P. H. Poulsen, K. A. Jørgensen, J. Am. Chem. Soc. 2013, 135, 8063–8070;
- 20bK. S. Halskov, T. Naicker, M. E. Jensen, K. A. Jørgensen, Chem. Commun. 2013, 49, 6382.
- 21Y. Demizu, M. Doi, Y. Sato, M. Tanaka, H. Okuda, M. Kurihara, J. Pept. Sci. 2011, 17, 420–426.
- 22K. Akagawa, K. Kudo, Adv. Synth. Catal. 2011, 353, 843–847.
- 23A. Weyer, D. Díaz, A. Nierth, N. E. Schlörer, A. Berkessel, ChemCatChem 2012, 4, 337–340.
- 24H. Maeda, S. Yamada, H. Itoh, Y. Hori, Chem. Commun. 2012, 48, 1772–1774.
- 25M. Stadler, B. List, Synlett 2008, 597–599.
- 26In this case, the ratio of products 3 and 5 was 14:86.
- 27
- 27aU. Matteoli, A. Ciappa, S. Bovo, M. Bertoldini, A. Scrivanti, Tetrahedron: Asymmetry 2007, 18, 797–802;
- 27bS. Superchi, V. Marchitiello, L. Pisani, P. Scafato, Chirality 2011, 23, 761–767.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.