Organocatalytic Asymmetric Synthesis of Propargylamines with Two Adjacent Stereocenters: Mannich-Type Reactions of In Situ Generated C-Alkynyl Imines with β-Keto Esters†
Dr. Taichi Kano
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Search for more papers by this authorDr. Taiga Yurino
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Keiji Maruoka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)Search for more papers by this authorDr. Taichi Kano
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Search for more papers by this authorDr. Taiga Yurino
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Keiji Maruoka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan)Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research from MEXT (Japan). T.Y. thanks the Japan Society for the Promotion of Science for Young Scientists for Research Fellowships.
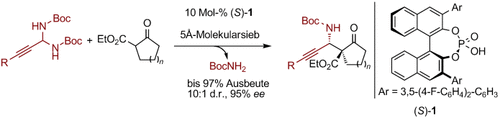
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201304963_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. A. Huffman, N. Yasuda, A. E. DeCamp, E. J. J. Grabowski, J. Org. Chem. 1995, 60, 1590;
- 1bM. Konishi, H. Ohkuma, T. Tsuno, T. Oki, G. D. VanDuyne, J. Clardy, J. Am. Chem. Soc. 1990, 112, 3715.
- 2For reviews, see:
- 2aA. E. Shilov, G. B. Shuĺpin, Chem. Rev. 1997, 97, 2879;
- 2bC. Wei, Z. Li, C.-J. Li, Synlett 2004, 1472;
- 2cP. G. Cozzi, R. Hilgraf, N. Zimmermann, Eur. J. Org. Chem. 2004, 4095;
- 2dH. J. Zhu, J. X. Jiang, J. Ren, Y. M. Yan, C. U. Pittman, Jr., Curr. Org. Synth. 2005, 2, 547;
- 2eL. Zani, C. Bolm, Chem. Commun. 2006, 4263;
- 2fM. Hatano, T. Miyamoto, K. Ishihara, Curr. Org. Chem. 2007, 11, 127;
- 2gB. M. Trost, A. H. Weiss, Adv. Synth. Catal. 2009, 351, 963.
- 3For representative examples on the catalytic asymmetric alkynylation of imines, see:
- 3aC. Wei, C.-J. Li, J. Am. Chem. Soc. 2002, 124, 5638;
- 3bN. Gommermann, C. Koradin, K. Polborn, P. Knochel, Angew. Chem. 2003, 115, 5941;
10.1002/ange.200352578 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5763;
- 3cJ. F. Traverse, A. H. Hoveyda, M. L. Snapper, Org. Lett. 2003, 5, 3273;
- 3dB. Jiang, Y.-G. Si, Angew. Chem. 2004, 116, 218; Angew. Chem. Int. Ed. 2004, 43, 216;
- 3eC. Wei, J. T. Mague, C.-J. Li, Proc. Natl. Acad. Sci. USA 2004, 101, 5749;
- 3fN. Gommermann, P. Knochel, Chem. Commun. 2004, 2324;
- 3gT. Knöpfel, P. Aschwanden, T. Ichikawa, T. Watanabe, E. M. Carreira, Angew. Chem. Int. Ed. 2004, 43, 6097; Angew. Chem. 2004, 116, 6097; Angew. Chem. Int. Ed. 2004, 43, 5971;
- 3hJ.-X. Ji, J. Wu, A. S. C. Chan, Proc. Natl. Acad. Sci. USA 2005, 102, 11196;
- 3iT. R. Wu, J. M. Chong, Org. Lett. 2006, 8, 15;
- 3jA. M. Taylor, S. L. Schreiber, Org. Lett. 2006, 8, 143;
- 3kA. Bisai, V. K. Singh, Org. Lett. 2006, 8, 2405;
- 3lP. Aschwanden, C. R. J. Stephenson, E. M. Carreira, Org. Lett. 2006, 8, 2437;
- 3mA. Z. Gonzalez, E. Canales, J. A. Soderquist, Org. Lett. 2006, 8, 3331;
- 3nF. Colombo, M. Benaglia, S. Orlandi, F. Usuelli, G. Celentano, J. Org. Chem. 2006, 71, 2064;
- 3oN. Gommermann, P. Knochel, Chem. Eur. J. 2006, 12, 4380;
- 3pA. W. Patterson, J. A. Ellman, J. Org. Chem. 2006, 71, 7110;
- 3qR. Dodda, C.-G. Zhao, Org. Lett. 2007, 9, 165;
- 3rL. Zani, T. Eichhorn, C. Bolm, Chem. Eur. J. 2007, 13, 2587;
- 3sM. Rueping, A. P. Antonchick, C. Brinkmann, Angew. Chem. 2007, 119, 7027;
10.1002/ange.200702439 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6903;
- 3tA. Labonne, L. Zani, L. Hintermann, C. Bolm, J. Org. Chem. 2007, 72, 5704;
- 3uS. Turcaud, F. Berhal, J. Royer, J. Org. Chem. 2007, 72, 7893;
- 3vG. Blay, L. Cardona, E. Climent, J. R. Pedro, Angew. Chem. 2008, 120, 5675;
10.1002/ange.200801020 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5593;
- 3wJ. A. Bishop, S. Lou, S. E. Schaus, Angew. Chem. 2009, 121, 4401; Angew. Chem. Int. Ed. 2009, 48, 4337;
- 3xS. Zhu, W. Yan, B. Mao, X. Jiang, R. Wang, J. Org. Chem. 2009, 74, 6980;
- 3yM. J. Campbell, F. D. Toste, Chem. Sci. 2011, 2, 1369.
- 4
- 4aN. S. Josephsohn, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2004, 126, 3734;
- 4bN. S. Josephsohn, E. L. Carswell, M. L. Snapper, A. H. Hoveyda, Org. Lett. 2005, 7, 2711;
- 4cH. Mandai, K. Mandai, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2008, 130, 17961;
- 4dM. Hatano, Y. Hattori, Y. Furuya, K. Ishihara, Org. Lett. 2009, 11, 2321.
- 5Representative reactive C-alkynyl imines:
- 5aH. Ishitani, S. Nagayama, S. Kobayashi, J. Org. Chem. 1996, 61, 1902;
- 5bP. B. Alper, C. Meyers, A. Lerchner, D. R. Siegel, E. M. Carreira, Angew. Chem. 1999, 111, 3379;
10.1002/(SICI)1521-3757(19991102)111:21<3379::AID-ANGE3379>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3186;10.1002/(SICI)1521-3773(19991102)38:21<3186::AID-ANIE3186>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 5cF. Ferreira, M. Audouin, F. Chemla, Chem. Eur. J. 2005, 11, 5269;
- 5dC. Roe, T. M. Solá, L. Sasraku-Neequaye, H. Hobbs, I. Churcher, D. MacPhersonc, R. A. Stockman, Chem. Commun. 2011, 47, 7491;
- 5eJ. Louvel, F. Chemla, E. Demont, F. Ferreira, A. Pérez-Luna, A. Voituriez, Adv. Synth. Catal. 2011, 353, 2137;
- 5fK. Banert, M. Hagedorn, A. Müller, Eur. J. Org. Chem. 2001, 1089;
- 5gP. Wipf, C. R. J. Stephenson, K. Okumura, J. Am. Chem. Soc. 2003, 125, 14694.
- 6T. Kano, T. Yurino, D. Asakawa, K. Maruoka, Angew. Chem. 2013, 125, 5642;
10.1002/ange.201300231 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 5532.
- 7For pioneering reports and a representative review on chiral phosphoric acid catalysts, see:
- 7aT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. 2004, 116, 1592;
10.1002/ange.200353240 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1566;
- 7bD. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356;
- 7cM. Terada, Synthesis 2010, 1929.
- 8For a related organocatalytic Mannich-type reaction between N-aryl-C-alkynyl imines and aldehydes, see: E. Gómez-Bengoa, J. Jiménez, I. Lapuerta, A. Mielgo, M. Oiarbide, I. Otazo, I. Velilla, S. Vera, C. Palomo, Chem. Sci. 2012, 3, 2949.
- 9For representative quaternary carbon construction through asymmetric Mannich reactions of imines with β-keto esters, see:
- 9aM. Marigo, A. Kjærsgaard, K. Juhl, N. Gathergood, K. A. Jørgensen, Chem. Eur. J. 2003, 9, 2359;
- 9bY. Hamashima, N. Sasamoto, D. Hotta, H. Somei, N. Umebayashi, M. Sodeoka, Angew. Chem. 2005, 117, 1549;
10.1002/ange.200462202 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1525;
- 9cT. B. Poulsen, C. Alemparte, S. Saaby, M. Bella, K. A. Jørgensen, Angew. Chem. 2005, 117, 2956;
10.1002/ange.200500144 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2896;
- 9dC. Foltz, B. Stecker, G. Marconi, S. Bellemin-Laponnaz, H. Wadepohl, L. H. Gade, Chem. Commun. 2005, 5115;
- 9eA. L. Tillman, J. Ye, D. J. Dixon, Chem. Commun. 2006, 1191;
- 9fA. Ting, S. Lou, S. E. Schaus, Org. Lett. 2006, 8, 2003;
- 9gY. Yamaoka, H. Miyabe, Y. Yasui, Y. Takemoto, Synthesis 2007, 2571;
- 9hO. Marianacci, G. Micheletti, L. Bernardi, F. Fini, M. Fochi, D. Pettersen, V. Sgarzani, A. Ricci, Chem. Eur. J. 2007, 13, 8338;
- 9iS. Lou, P. Dai, S. E. Schaus, J. Org. Chem. 2007, 72, 9998;
- 9jZ. Chen, H. Morimoto, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2008, 130, 2170;
- 9kY. Hamashima, N. Sasamoto, N. Umebayashi, M. Sodeoka, Chem. Asian J. 2008, 3, 1443;
- 9lY. K. Kang, D. Y. Kim, J. Org. Chem. 2009, 74, 5734;
- 9mM. Hatano, T. Horibe, K. Ishihara, J. Am. Chem. Soc. 2010, 132, 56;
- 9nX. Jiang, D. Fu, G. Zhang, Y. Cao, L. Liu, J. Song, R. Wang, Chem. Commun. 2010, 46, 4294;
- 9oM. Hatano, K. Moriyama, T. Maki, K. Ishihara, Angew. Chem. 2010, 122, 3911;
10.1002/ange.201000824 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3823;
- 9pZ. Liu, M. Shi, Organometallics 2010, 29, 2831;
- 9qG. Zhang, Y. Zhang, R. Wang, Angew. Chem. 2011, 123, 10613; Angew. Chem. Int. Ed. 2011, 50, 10429;
- 9rW. Yan, D. Wang, J. Feng, P. Li, D. Zhao, R. Wang, Org. Lett. 2012, 14, 2512.
- 10Under identical reaction conditions, copper (II) trifluoromethanesulfonate (Cu(OTf)2) as a Lewis acid catalyst also gave the adduct 7 a in good yield (97 %) with low diastereoselectivity (anti/syn=1:1.3).
- 11CCDC 942074 (8) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.