Rhodium-Catalyzed Oxidative Annulation of Sulfoximines and Alkynes as an Approach to 1,2-Benzothiazines†
Wanrong Dong
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorLong Wang
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorFangfang Pan
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Carsten Bolm
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)Search for more papers by this authorWanrong Dong
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorLong Wang
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorFangfang Pan
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Carsten Bolm
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)Search for more papers by this authorW.D. and F.P. thank the China Scholarship Council for predoctoral stipends. K.P. acknowledges support by the Alexander von Humboldt Foundation. We also thank Prof. Dr. J. Cheng (Changzhou University) for helpful discussions and Prof. Dr. U. Englert as well as Dr. R. Wang (both RWTH Aachen University) for the collection of X-ray crystallographic data.
Graphical Abstract
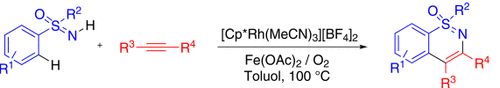
Revitalisierung durch Sauerstoff: Eine Rhodium(III)-katalysierte Sequenz aus oxidativer C-H/N-H-Aktivierung und Anellierung eröffnet den Zugang zu substituierten 1,2-Benzothiazinen ausgehend von leicht verfügbaren NH-Sulfoximinen und Alkinen (siehe Schema; Cp*=C5Me5). Das Oxidationssystem besteht aus molekularem Sauerstoff und einer katalytischen Menge Fe(OAc)2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201304456_sm_miscellaneous_information.pdf8.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212;
- 1bJ. Wencel-Delord, T. Droge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740;
- 1cG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 1dP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879;
- 1eL. Ackermann, Acc. Chem. Res. 2013, DOI: .
- 2
- 2aZ. Shi, C. Zhang, S. Li, D. Pan, S. Ding, Y. Cui, N. Jiao, Angew. Chem. 2009, 121, 4642; Angew. Chem. Int. Ed. 2009, 48, 4572;
- 2bS. Ding, Z. Shi, N. Jiao, Org. Lett. 2010, 12, 1540;
- 2cZ. Shi, Y. Cui, N. Jiao, Org. Lett. 2010, 12, 2908;
- 2dJ. Chen, Q. Pang, Y. Sun, X. Li, J. Org. Chem. 2011, 76, 3523;
- 2eR. R. Suresh, K. C. Kumara Swamy, J. Org. Chem. 2012, 77, 6959;
- 2fL. Wang, J. Huang, S. Peng, H. Liu, X. Jiang, J. Wang, Angew. Chem. 2013, 125, 1812; Angew. Chem. Int. Ed. 2013, 52, 1768;
- 2gM. R. Kuram, M. Bhanuchandra, A. K. Sahoo, Angew. Chem. 2013, 125, 4705;
10.1002/ange.201210217 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4607.
- 3For selected examples of RhIII-catalyzed annulations, see:
- 3aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407;
- 3bN. Umeda, H. Tsurugi, T. Satoh, M. Miura, Angew. Chem. 2008, 120, 4083;
10.1002/ange.200800924 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4019;
- 3cD. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 16474;
- 3dS. Mochida, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2009, 74, 6295;
- 3eT. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141;
- 3fP. Too, Y.-F. Wang, S. Chiba, Org. Lett. 2010, 12, 5688;
- 3gY. Su, M. Zhao, K. Han, G. Song, X. Li, Org. Lett. 2010, 12, 5462;
- 3hJ. Chen, G. Song, C. Pan, X. Li, Org. Lett. 2010, 12, 5426;
- 3iK. Morimoto, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2010, 12, 2068;
- 3jD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326;
- 3kT. K. Hyster, T. Rovis, J. Am. Chem. Soc. 2010, 132, 10565;
- 3lS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 3mN. Guimond, C. Gouliaras, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 6908;
- 3nS. Mochida, M. Shimizu, K. Hirano, T. Satoh, M. Miura, Chem. Asian J. 2010, 5, 847;
- 3oX. Wei, M. Zhao, Z. Du, X. Li, Org. Lett. 2011, 13, 4636;
- 3pG. Song, X. Gong, X. Li, J. Org. Chem. 2011, 76, 7583;
- 3qP. C. Too, S. H. Chua, S. H. Wong, S. Chiba, J. Org. Chem. 2011, 76, 6159;
- 3rN. Guimond, S. I. Gorelsky, K. Fagnou, J. Am. Chem. Soc. 2011, 133, 6449;
- 3sX. Zhang, D. Chen, M. Zhao, J. Zhao, A. Jia, X. Li, Adv. Synth. Catal. 2011, 353, 719;
- 3tJ. Jayakumar, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2012, 124, 201;
10.1002/ange.201105755 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 197;
- 3uL. Zheng, J. Ju, Y. Bin, R. Hua, J. Org. Chem. 2012, 77, 5794;
- 3vH. Wang, F. Glorius, Angew. Chem. 2012, 124, 7430; Angew. Chem. Int. Ed. 2012, 51, 7318;
- 3wH. Wang, C. Grohmann, C. Nimphius, F. Glorius, J. Am. Chem. Soc. 2012, 134, 19592;
- 3xX. Xu, Y. Liu, C.-M. Park, Angew. Chem. 2012, 124, 9506; Angew. Chem. Int. Ed. 2012, 51, 9372;
- 3yM. Presset, D. Oehlrich, F. Rombouts, G. A. Molander, Org. Lett. 2013, 12, 5426;
- 3zJ.-R. Huang, Q.-R. Zhang, C.-H. Qu, X.-H. Sun, L. Dong, Y.-C. Chen, Org. Lett. 2013, 15, 1878.
- 4aL. Ackermann, A. V. Lygin, N. Hofmann, Angew. Chem. 2011, 123, 6503; Angew. Chem. Int. Ed. 2011, 50, 6379;
- 4bB. Li, H. Feng, S. Xu, B. Wang, Chem. Eur. J. 2011, 17, 12573;
- 4cL. Ackermann, A. V. Lygin, N. Hofmann, Org. Lett. 2011, 13, 3278;
- 4dL. Ackermann, S. Fenner, Org. Lett. 2011, 13, 6548;
- 4eR. K. Chinnagolla, M. Jeganmohan, Chem. Commun. 2012, 48, 2030;
- 4fL. Ackermann, L. Wang, A. V. Lygin, Chem. Sci. 2012, 3, 177;
- 4gK. R. Chinnagolla, M. Jeganmohan, Eur. J. Org. Chem. 2012, 417;
- 4hL. Ackermann, A. V. Lygin, Org. Lett. 2012, 14, 764;
- 4iL. Ackermann, J. Pospech, K. Graczyk, K. Rauch, Org. Lett. 2012, 14, 930;
- 4jR. K. Chinnagolla, S. Pimparkar, M. Jeganmohan, Org. Lett. 2012, 14, 3032;
- 4kV. S. Thirunavukkarasu, M. Donati, L. Ackermann, Org. Lett. 2012, 14, 3416;
- 4lK. Parthasarathy, N. Senthilkumar, J. Jayakumar, C.-H. Cheng, Org. Lett. 2012, 14, 3478;
- 4mB. Li, H. Feng, N. Wang, J. Ma, H. Song, S. Xu, B. Wang, Chem. Eur. J. 2012, 18, 12873;
- 4nS. Reddy Chidipudi, I. Khan, H. W. Lam, Angew. Chem. 2012, 124, 12281;
10.1002/ange.201207170 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12115;
- 4oL. Wang, L. Ackermann, Org. Lett. 2013, 15, 176.
- 5
- 5aT. R. Williams, D. J. Cram, J. Org. Chem. 1973, 38, 20;
- 5bP. Stoss, G. Satzinger, Chem. Ber. 1972, 105, 2575;
- 5cT. R. Williams, D. J. Cram, J. Am. Chem. Soc. 1971, 93, 7333;
- 5dJ. G. Lombardino, D. E. Kuhla, Adv. Heterocycl. Chem. 1981, 28, 73.
- 6
- 6aM. Harmata, X. Hong, C. L. Barnes, Tetrahedron Lett. 2003, 44, 7261;
- 6bM. Harmata, X. Hong, C. L. Barnes, Org. Lett. 2004, 6, 2201;
- 6cM. Harmata, X. Hong, Tetrahedron Lett. 2005, 46, 3847.
- 7
- 7aM. Harmata, M. Kahraman, D. E. Jones, N. Pavri, S. E. Weatherwax, Tetrahedron 1998, 54, 9995, and references therein.
- 8
- 8aM. Harmata, N. Pavri, Angew. Chem. 1999, 111, 2577;
10.1002/(SICI)1521-3757(19990816)111:16<2577::AID-ANGE2577>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2419;10.1002/(SICI)1521-3773(19990816)38:16<2419::AID-ANIE2419>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 8bM. Harmata, S. K. Ghosh, Org. Lett. 2001, 3, 3321;
- 8cM. Harmata, X. Hong, J. Am. Chem. Soc. 2003, 125, 5754;
- 8dM. Harmata, S. K. Ghosh, C. L. Barnes, J. Supramol. Chem. 2003, 2, 349;
- 8eM. Harmata, N. L. Calkins, R. G. Banghman, C. L. Barnes, J. Org. Chem. 2006, 71, 3650.
- 9M. Harmata, K. Rayanil, M. G. Gomes, P. Zheng, N. L. Calkins, S.-Y. Kim, Y. Fan, V. Bumbu, D. R. Lee, S. Wacharasindhu, X. Hong, Org. Lett. 2005, 7, 143.
- 10aM. Miyasaka, K. Hirano, T. Satoh, R. Kowalczyk, C. Bolm, M. Miura, Org. Lett. 2011, 13, 359;
- 10bL. Wang, H. Huang, D. L. Priebbenow, F.-F. Pan, C. Bolm, Angew. Chem. 2013, 125, 3562; Angew. Chem. Int. Ed. 2013, 52, 3478.
- 11M. V. Pham, B. Ye, N. Cramer, Angew. Chem. 2012, 124, 10762; Angew. Chem. Int. Ed. 2012, 51, 10610.
- 12CCDC 937990 (3 n) and 949306 (rhodacycle VI) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13
- 13aM. Catellani, Top. Organomet. Chem. 2005, 8, 21;
10.1007/b104126 Google Scholar
- 13bM. Catellani, E. Motti, N. D. Ca, Acc. Chem. Res. 2008, 41, 1512;
- 13cC.-H. Jun, C. W. Moon, D.-Y. Lee, Chem. Eur. J. 2002, 8, 2422;
10.1002/1521-3765(20020603)8:11<2422::AID-CHEM2422>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 13dY. J. Park, C.-H. Jun, Bull. Korean Chem. Soc. 2005, 26, 877;
- 13eL. Ackermann, R. Vicente, Top. Curr. Chem. 2009, 292, 211;
- 13fK. Fagnou, Top. Curr. Chem. 2009, 292, 35;
- 13gX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 13hD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 13iT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 13jS. H. Cho, J. Y. Kim, J. Kwak, S. Chang, Chem. Soc. Rev. 2011, 40, 5068.
- 14T. K. Hyster, T. Rovis, Chem. Sci. 2011, 2, 1606.
- 15E. M. Simmons, J. F. Hartwig, Angew. Chem. 2012, 124, 3120;
10.1002/ange.201107334 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3066, and references therein.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.