Tetrahedral Anion Cage: Self-Assembly of a (PO4)4L4 Complex from a Tris(bisurea) Ligand†
Corresponding Author
Prof. Biao Wu
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)Search for more papers by this authorDr. Fengjuan Cui
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorDr. Yibo Lei
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDr. Shaoguang Li
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorDr. Nader de Sousa Amadeu
Institut für Anorganische Chemie und Strukturchemie, Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf (Germany)
Search for more papers by this authorProf. Dr. Christoph Janiak
Institut für Anorganische Chemie und Strukturchemie, Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf (Germany)
Search for more papers by this authorProf. Yue-Jian Lin
Shanghai Key Laboratory of Molecular Catalysis and Innovative Material, Department of Chemistry, Fudan University, Shanghai, 200433 (China)
Search for more papers by this authorProf. Lin-Hong Weng
Shanghai Key Laboratory of Molecular Catalysis and Innovative Material, Department of Chemistry, Fudan University, Shanghai, 200433 (China)
Search for more papers by this authorProf. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Xiao-Juan Yang
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorCorresponding Author
Prof. Biao Wu
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)Search for more papers by this authorDr. Fengjuan Cui
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorDr. Yibo Lei
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDr. Shaoguang Li
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorDr. Nader de Sousa Amadeu
Institut für Anorganische Chemie und Strukturchemie, Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf (Germany)
Search for more papers by this authorProf. Dr. Christoph Janiak
Institut für Anorganische Chemie und Strukturchemie, Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf (Germany)
Search for more papers by this authorProf. Yue-Jian Lin
Shanghai Key Laboratory of Molecular Catalysis and Innovative Material, Department of Chemistry, Fudan University, Shanghai, 200433 (China)
Search for more papers by this authorProf. Lin-Hong Weng
Shanghai Key Laboratory of Molecular Catalysis and Innovative Material, Department of Chemistry, Fudan University, Shanghai, 200433 (China)
Search for more papers by this authorProf. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Xiao-Juan Yang
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, CAS, Lanzhou 730000 (China)
Search for more papers by this authorThis work was supported by the National Natural Science Foundation of China (Grant No. 21271149).
Graphical Abstract
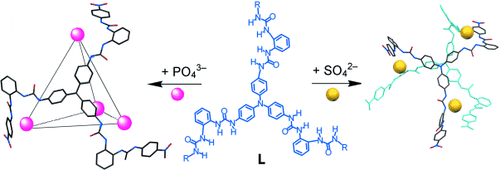
Der C3-symmetrische Tris(diharnstoff)-Ligand L bildet gemeinsam mit PO43−-Ionen den tetraedrischen Käfig [(PO4)4L4]12−. Die PO43−-Ionen an den vier Ecken dieses Käfigs bilden jeweils 12 NH⋅⋅⋅O-Wasserstoffbrücken mit 6 Harnstoffgruppen. Mit SO42−-Ionen schließt sich L zu dem beispiellosen windradförmigen Dreikernkomplex [(SO4)3L2]6− zusammen (siehe Bild).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209930_sm_miscellaneous_information.pdf2.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected books and reviews on anion binding, see
- 1aJ. L. Sessler, P. A. Gale, W.-S. Cho, Anion Receptor Chemistry, Cambridge, 2006;
10.1039/9781847552471 Google Scholar
- 1bP. D. Beer, P. A. Gale, Angew. Chem. 2001, 113, 502–532;
10.1002/1521-3757(20010202)113:3<502::AID-ANGE502>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 486–516;10.1002/1521-3773(20010202)40:3<486::AID-ANIE486>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 1cP. A. Gale, Acc. Chem. Res. 2006, 39, 465–475;
- 1dE. A. Katayev, Y. A. Ustynyuk, J. L. Sessler, Coord. Chem. Rev. 2006, 250, 3004–3037;
- 1eP. A. Gale, S. E. García-Garrido, J. Garric, Chem. Soc. Rev. 2008, 37, 151–190;
- 1ffor latest reviews on anion chemistry, see a thematic issue: Chem. Soc. Rev. 2010, 3581–4008.
- 2
- 2aJ.-M. Lehn, Acc. Chem. Res. 1978, 11, 49–57;
- 2bJ.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995;
10.1002/3527607439 Google Scholar
- 2cK. Bowman-James, Acc. Chem. Res. 2005, 38, 671–678;
- 2d Anion Coordination Chemistry (Eds.: ) Wiley-VCH, Weinheim, 2011.
- 3
- 3aI. M. Oppel (née Müller), K. Föcker, Angew. Chem. 2008, 120, 408–411;
10.1002/ange.200703789 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 402–405;
- 3bI. M. Müller, D. Möller, C. A. Schalley, Angew. Chem. 2005, 117, 485–488; Angew. Chem. Int. Ed. 2005, 44, 480–484;
- 3cJ. F. Bai, A. V. Virovets, M. Scheer, Science 2003, 300, 781–783;
- 3dQ.-F. Sun, J. Iwasa, D. Ogawa, Y. Ishido, S. Sato, T. Ozeki, Y. K. Yamaguchi, M. Fujita, Science 2010, 328, 1144–1147.
- 4
- 4aH. Amouri, C. Desmarets, J. Moussa, Chem. Rev. 2012, 112, 2015–2041;
- 4bR. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918;
- 4cS. J. Dalgarno, N. P. Power, J. L. Atwood, Coord. Chem. Rev. 2008, 252, 825–841.
- 5For selected reviews and articles, see
- 5aM. D. Pluth, R. G. Bergman, K. N. Raymond, Science 2007, 316, 85–88;
- 5bP. Mal, B. Breiner, K. Rissanen, J. R. Nitschke, Science 2009, 324, 1697–1699;
- 5cT. S. Koblenz, J. Wassenaar, J. N. H. Reek, Chem. Soc. Rev. 2008, 37, 247–262;
- 5dL. Trembleau, J. Rebek, Jr., Science 2003, 301, 1219–1221;
- 5eM. D. Ward, Chem. Commun. 2009, 4487–4499;
- 5fD. L. Caulder, K. N. Raymond, Acc. Chem. Res. 1999, 32, 975–982.
- 6For examples of M4L6 cages, see
- 6aR. W. Saalfrank, A. Stark, K. Peters, H. G. von Schnering, Angew. Chem. 1988, 100, 878–880; Angew. Chem. Int. Ed. Engl. 1988, 27, 851–853;
- 6bM. D. Pluth, R. G. Bergman, K. N. Raymond, Acc. Chem. Res. 2009, 42, 1650–1659, and references therein;
- 6cR. L. Paul, Z. R. Bell, J. C. Jeffery, J. A. McCleverty, M. D. Ward, Proc. Natl. Acad. Sci. USA 2002, 99, 4883–4888;
- 6dN. Ousaka, J. K. Clegg, J. R. Nitschke, Angew. Chem. 2012, 124, 1493–1497;
10.1002/ange.201107532 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1464–1468.
- 7For examples of M4L4 cages, see
- 7aA. J. Amoroso, J. C. Jeffery, P. L. Jones, J. A. McCleverty, P. Thornton, M. D. Ward, Angew. Chem. 1995, 107, 1577–1580;
10.1002/ange.19951071314 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1443–1446;
- 7bD. L. Caulder, C. Bruckner, R. E. Powers, S. Konig, T. N. Parac, J. A. Leary, K. N. Raymond, J. Am. Chem. Soc. 2001, 123, 8923–8938;
- 7cB. Birkmann, R. Fröhlich, F. E. Hahn, Chem. Eur. J. 2009, 15, 9325–9329.
- 8
- 8aE. Graf, J.-M. Lehn, J. Am. Chem. Soc. 1975, 97, 5022–5024;
- 8bF. P. Schmidtchen, Angew. Chem. 1977, 89, 751–752; Angew. Chem. Int. Ed. Engl. 1977, 16, 720–721;
- 8cK. Ichikawa, M. A. Hossain, Chem. Commun. 1996, 1721–1722;
- 8dQ.-Q. Wang, V. W. Day, K. Bowman-James, Angew. Chem. 2012, 124, 2161–2165; Angew. Chem. Int. Ed. 2012, 51, 2119–2123;
- 8eQ.-Q. Wang, V. W. Day, K. Bowman-James, J. Am. Chem. Soc. 2013, 135, 392–399.
- 9
- 9aM. Lankshear, P. D. Beer, Coord. Chem. Rev. 2006, 250, 3142–3160;
- 9bB. Hasenknopf, J.-M. Lehn, G. Baum, B. O. Kneisel, D. Fenske, Angew. Chem. 1996, 108, 1987–1990;
10.1002/ange.19961081632 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1838–1840;
- 9cJ. S. Fleming, K. L. V. Mann, C.-A. Carraz, E. Psillakis, J. C. Jeffery, J. A. McCleverty, M. D. Ward, Angew. Chem. 1998, 110, 1315–1318;
10.1002/(SICI)1521-3757(19980504)110:9<1315::AID-ANGE1315>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1279–1281;10.1002/(SICI)1521-3773(19980518)37:9<1279::AID-ANIE1279>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 9dB. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. Van Dorsselaer, B. O. Kneisel, D. Fenske, J. Am. Chem. Soc. 1997, 119, 10956–10962.
- 10
- 10aK. M. Mullen, P. D. Beer, Chem. Soc. Rev. 2009, 38, 1701–1713;
- 10bM. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul, A. R. Cowley, F. Szemes, M. G. B. Drew, J. Am. Chem. Soc. 2005, 127, 2292–2302;
- 10cH. Juwarker, K.-S. Jeong, Chem. Soc. Rev. 2010, 39, 3664–3674;
- 10dJ. Sánchez-Quesada, C. Seel, P. Prados, J. de Mendoza, J. Am. Chem. Soc. 1996, 118, 277–278;
- 10eJ. Keegan, P. E. Kruger, M. Nieuwenhuyzen, J. O’Brien, N. Martin, Chem. Commun. 2001, 2192–2193;
- 10fS. J. Coles, J. G. Frey, P. A. Gale, M. B. Hurst-House, M. E. Light, K. Navakhun, G. L. Thomas, Chem. Commun. 2003, 568–569;
- 10gR. Custelcean, P. V. Bonnesen, B. D. Roach, N. C. Duncan, Chem. Commun. 2012, 48, 7438–7440.
- 11
- 11aC. Jia, B. Wu, S. Li, Z. Yang, Q. Zhao, J. Liang, Q.-S. Li, X.-J. Yang, Chem. Commun. 2010, 46, 5376–5378;
- 11bC. Jia, B. Wu, S. Li, X. Huang, X.-J. Yang, Org. Lett. 2010, 12, 5612–5615;
- 11cC. Jia, B. Wu, S. Li, X. Huang, Q. Zhao, Q.-S. Li, X.-J. Yang, Angew. Chem. 2011, 123, 506–510; Angew. Chem. Int. Ed. 2011, 50, 486–490;
- 11dS. Li, C. Jia, B. Wu, Q. Luo, X. Huang, Z. Yang, Q.-S. Li, X.-J. Yang, Angew. Chem. 2011, 123, 5839–5842; Angew. Chem. Int. Ed. 2011, 50, 5721–5724.
- 12
- 12aB. P. Hay, T. K. Firman, B. A. Moyer, J. Am. Chem. Soc. 2005, 127, 1810–1819;
- 12bR. Custelcean, B. A. Moyer, B. P. Hay, Chem. Commun. 2005, 5971–5973;
- 12cR. Custelcean, P. Remy, P. V. Bonnesen, D.-E. Jiang, B. A. Moyer, Angew. Chem. 2008, 120, 1892–1896;
10.1002/ange.200704937 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1866–1870.
- 13CCDC 909133 (1) and 909134 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14The structure of the tetrahedral cage [(PO4)4L4]12− was optimized at the B3LYP/6-31G level using the Gaussian 03 program suit (for full citation, see the Supporting Information).
- 15The cavity volume was calculated using the VOIDOO program, with a 1.4 Å diameter rolling ball on the crystal structure of complex 1.
- 16
- 16aS. Mecozzi, J. Rebek, Jr., Chem. Eur. J. 1998, 4, 1016–1022;
10.1002/(SICI)1521-3765(19980615)4:6<1016::AID-CHEM1016>3.0.CO;2-B CAS Web of Science® Google Scholar
- 16bR. A. Bilbeisi, J. K. Clegg, N. Elgrishi, X. de Hatten, M. Devillard, B. Breiner, P. Mal, J. R. Nitschke, J. Am. Chem. Soc. 2012, 134, 5110–5119.
- 17B. Conerney, P. Jensen, P. E. Kruger, C. MacGloinn, Chem. Commun. 2003, 1274–1275.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.