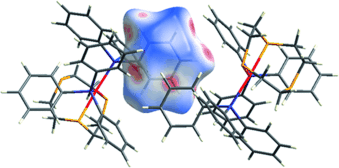
Bowing to the Pressure of π⋅⋅⋅π Interactions: Bending of Phenyl Rings in a Palladium(II) Thioether Crown Complex†
Henry L. S. Wong
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorDr. David R. Allan
Diamond Light Source, Harwell Science and Innovation Campus, Didcot (UK)
Search for more papers by this authorProf. Dr. Neil R. Champness
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorDr. Jonathan McMaster
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Schröder
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)Search for more papers by this authorCorresponding Author
Prof. Dr. Alexander J. Blake
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)Search for more papers by this authorHenry L. S. Wong
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorDr. David R. Allan
Diamond Light Source, Harwell Science and Innovation Campus, Didcot (UK)
Search for more papers by this authorProf. Dr. Neil R. Champness
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorDr. Jonathan McMaster
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Schröder
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)Search for more papers by this authorCorresponding Author
Prof. Dr. Alexander J. Blake
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)
School of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD (UK)Search for more papers by this authorWe thank the EPSRC and the University of Nottingham for funding and Diamond Light Source for the award of beamtime. M.S. gratefully acknowledges an ERC Advanced Grant and N.R.C. a Royal Society Wolfson Merit Award. We are also grateful to one referee for detailed comments and suggestions.
Graphical Abstract
Das Komprimieren einer parallelen vierfachen Phenylwechselwirkung zweier Kationen in [Pd([9]aneS3)(PPh3)2](PF6)2 ([9]aneS3=1,4,7-Trithiacyclononan) führt zu π⋅⋅⋅π-Stapelwechselwirkungen und einer begleitenden Pyramidalisierung am ipso-C-Atom eines der Phenylringe. Diese durch Druck ausgelösten Änderungen wurden mithilfe einer Hirshfeld-Oberflächenanalyse, Dichtefunktionalrechnungen und einer NBO-Analyse erklärt.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209845_sm_miscellaneous_information.pdf2.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. A. Hunter, J. K. M. Sanders, J. Am. Chem. Soc. 1990, 112, 5525–5534; C. A. Janiak, J. Chem. Soc. Dalton Trans. 2000, 3885–3896; S. Tsuzuki, K. Honda, T. Uchimaru, M. Mikami, K. Tanabe, J. Am. Chem. Soc. 2002, 124, 104–112; R. K. Raju, J. W. G. Bloom, Y. An, S. E. Wheeler, ChemPhysChem 2011, 12, 3116–3130.
- 2L. M. Salonen, M. Ellermann, F. Diederich, Angew. Chem. 2011, 123, 4908–4944;
10.1002/ange.201007560 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4808–4842.
- 3E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. 2003, 115, 1244–1287; Angew. Chem. Int. Ed. 2003, 42, 1210–1250.
- 4K. Müller-Dethlefs, P. Hobza, Chem. Rev. 2000, 100, 143–167.
- 5H. W. Roesky, M. Andruh, Coord. Chem. Rev. 2003, 236, 91–119.
- 6J. Jia, P. Hubberstey, N. R. Champness, M. Schröder, Struct. Bonding (Berlin) 2009, 132, 135–161.
- 7S. S. Babu, H. Möhwald, T. Nakanishi, Chem. Soc. Rev. 2010, 39, 4021–4035.
- 8J. K. Klosterman, Y. Yamauchi, M. Fujita, Chem. Soc. Rev. 2009, 38, 1714–1725.
- 9M. X. Wang, Acc. Chem. Res. 2012, 45, 182–195.
- 10T. Hasell, X. Wu, J. T. A. Jones, J. Bacsa, A. Steiner, T. Mitra, A. Trewin, D. J. Adams, A. I. Cooper, Nat. Chem. 2010, 2, 750–755.
- 11R. Fourme, E. Girard, R. Kahn, A.-C. Dhaussy, I. Ascone, Annu. Rev. Biophys. 2009, 38, 153–171.
- 12E. V. Boldyreva, Acta Crystallogr. Sect. A 2008, 64, 218–231.
- 13A. Katrusiak, Acta Crystallogr. Sect. A 2008, 64, 135–148.
- 14D. R. Allan, A. J. Blake, D. Huang, T. J. Prior, M. Schröder, Chem. Commun. 2006, 4081–4083.
- 15A. J. Blake, Y. V. Roberts, M. Schröder, J. Chem. Soc. Dalton Trans. 1996, 1885–1896.
- 16A. J. Blake, M. Schröder, Adv. Inorg. Chem. 1990, 35, 1–81.
- 17A. J. Blake, A. J. Holder, Y. V. Roberts, M. Schröder, Acta Crystallogr. Sect. C 1988, 44, 360–361.
- 18I. Dance, M. Scudder, CrystEngComm 2009, 11, 2233–2247.
- 19Cambridge Crystallographic Data Centre. Cambridge Structural Database, Version of November 2012. CCDC, 12 Union Road, Cambridge, CB2 1EW, UK.
- 20The use of a diamond anvil cell for high-pressure structure determination severely limits the fraction of total diffraction data that can be accessed, in this case to 21.5–31.4 %. The consequences of this include the need for extensive constraints and restraints during refinement (see the Supporting Information) and for particular care in interpreting the resulting geometric parameters and their precision.
- 21A decrease in unit-cell volume of approximately 25 % over a pressure range of about 75 kbar appears to be typical for coordination complexes (see for example entries 14, 22 and 28 in Table S1 of the Supporting Information).
- 22M. A. Spackman, D. Jayatilaka, CrystEngComm 2009, 11, 19–32.
- 23P. A. Wood, J. J. McKinnon, S. Parsons, E. Pidcock, M. A. Spackman, CrystEngComm 2008, 10, 368–376.
- 24CCDC 895226, 895227, 895228, 895229, 895230, 895231, 895232 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.