Enantioselective Synthesis of Epoxides Having a Tetrasubstituted Trifluoromethylated Carbon Center: Methylhydrazine-Induced Aerobic Epoxidation of β,β-Disubstituted Enones†
Hiroyuki Kawai
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorSatoshi Okusu
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorZhe Yuan
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorEtsuko Tokunaga
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorAkihito Yamano
Rigaku Corporation, 3-9-12 Mastubara-cho, Akishima Tokyo 196-8666 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Mastubara-cho, Akishima Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Norio Shibata
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)===Search for more papers by this authorHiroyuki Kawai
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorSatoshi Okusu
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorZhe Yuan
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorEtsuko Tokunaga
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorAkihito Yamano
Rigaku Corporation, 3-9-12 Mastubara-cho, Akishima Tokyo 196-8666 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Mastubara-cho, Akishima Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Norio Shibata
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)===Search for more papers by this authorThis study was financially supported in part by Grants-in-Aid for Scientific Research from the MEXT (Ministry of Education, Culture, Sports, Science and Technology) (24105513, Project No. 2304: Advanced Molecular Transformation by Organocatalysts). We thank the Asahi Glass Foundation for partial support. E.T. acknowledges Grants-in-Aid for Scientific Research for financial support (24915016). We also thank INSA de Rouen, France for providing an internship for Z.Y.
Graphical Abstract
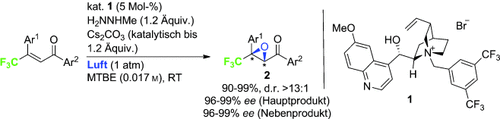
Aus der Luft gegriffen: Die neuartige Titelreaktion wird von einem System aus Methylhydrazin, einer Base und dem Organokatalysator 1 katalysiert. Biologisch attraktive Epoxide 2 mit tetrasubstituiertem trifluormethyliertem Kohlenstoffzentrum wurden mit exzellenter Enantioselektivität erhalten. 18O-Markierungsexperimente deuten darauf hin, dass bei der Reaktion molekularer Sauerstoff aktiviert wird. MTBE=Methyl-tert-butylether.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209355_sm_miscellaneous_information.pdf5.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Katsuki, K. B. Sharpless, J. Am. Chem. Soc. 1980, 102, 5974–5976.
- 2
- 2aM. J. Porter, J. Skidmore, Chem. Commun. 2000, 1215–1225;
- 2bQ.-H. Xia, H.-Q. Ge, C.-P. Ye, Z.-M. Liu, K.-X. Su, Chem. Rev. 2005, 105, 1603–1662;
- 2cO. A. Wong, Y. Shi, Chem. Rev. 2008, 108, 3958–3987;
- 2dK. Matsumoto, T. Katsuki in Catalytic Asymmetric Synthesis, 3rd ed. ), Wiley-VCH, New York, 2010.
- 3
- 3aW. Zhang, J. L. Loebach, S. R. Wilson, E. N. Jacobsen, J. Am. Chem. Soc. 1990, 112, 2801–2803;
- 3bR. Irie, K. Noda, Y. Ito, N. Matsumoto, T. Katsuki, Tetrahedron Lett. 1990, 31, 7345–7348;
- 3cM. Bougauchi, S. Watanabe, T. Arai, H. Sasai, M. Shibasaki, J. Am. Chem. Soc. 1997, 119, 2329–2330;
- 3dW. Zhang, H. Yamamoto, J. Am. Chem. Soc. 2007, 129, 286–287;
- 3eK. Matsumoto, T. Oguma, T. Katsuki, Angew. Chem. 2009, 121, 7568–7571; Angew. Chem. Int. Ed. 2009, 48, 7432–7435.
- 4
- 4aT. Mukaiyama, T. Yamada, T. Nagata, K. Imagawa, Chem. Lett. 1993, 327–330;
- 4bD. Enders, J. Zhu, G. Raabe, Angew. Chem. 1996, 108, 1827–1829;
10.1002/ange.19961081530 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1725–1728;
- 4cT.-S. Lai, R. Zhang, K.-K. Cheung, H.-L. Kwong, C.-M. Che, Chem. Commun. 1998, 1583–1584;
- 4dH. Tanaka, H. Nishikawa, T. Uchida, T. Katsuki, J. Am. Chem. Soc. 2010, 132, 12034–12041;
- 4eS. Koya, Y. Nishioka, H. Mizoguchi, T. Uchida, T. Katsuki, Angew. Chem. 2012, 124, 8368–8371;
10.1002/ange.201201848 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 8243–8246.
- 5
- 5aX.-Y. Wu, X. She, Y. Shi, J. Am. Chem. Soc. 2002, 124, 8792–8793;
- 5bT. Ooi, D. Ohara, M. Tamura, K. Maruoka, J. Am. Chem. Soc. 2004, 126, 6844–6845;
- 5cM. Marigo, J. Franzen, T. B. Poulsen, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 6964–6965;
- 5dB. F. Sun, B. Cindric, L. Deng, J. Am. Chem. Soc. 2008, 130, 8134–8135;
- 5eX. Wang, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2008, 130, 6070–6071.
- 6
- 6aW. Adam, P. B. Rao, H.-G. Degen, C. R. Saha-Möller, Eur. J. Org. Chem. 2002, 630–639;
- 6bY. Nishikawa, H. Yamamoto, J. Am. Chem. Soc. 2011, 133, 8432–8435.
- 7Y. Chu, X. Liu, W. Li, X. Hu, L. Lin, X. Feng, Chem. Sci. 2012, 3, 1996–2000.
- 8
- 8aJ. T. Welch, S. Eswarakrishman, Fluorine in Bioorganic Chemistry, Wiley, New York, 1991;
10.1021/bk-1991-0456 Google Scholar
- 8bP. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 8cK. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, UK, 2006;
10.1002/9780470988589 Google Scholar
- 8dK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 8eI. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, UK, 2009.
10.1002/9781444312096 Google Scholar
- 9
- 9aS. Mizuta, N. Shibata, S. Akiti, H. Fujimoto, S. Nakamura, T. Toru, Org. Lett. 2007, 9, 3707–3710;
- 9bS. Ogawa, N. Shibata, J. Inagaki, S. Nakamura, T. Toru, Angew. Chem. 2007, 119, 8820–8823;
10.1002/ange.200703317 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8666–8669;
- 9cH. Kawai, A. Kusuda, S. Nakamura, M. Shiro, N. Shibata, Angew. Chem. 2009, 121, 6442–6445; Angew. Chem. Int. Ed. 2009, 48, 6324–6327;
- 9dY. Huang, E. Tokunaga, S. Suzuki, M. Shiro, N. Shibata, Org. Lett. 2010, 12, 1136–1138;
- 9eH. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Org. Lett. 2010, 12, 5104–5107;
- 9fA. Matsnev, S. Noritake, Y. Nomura, E. Tokunaga, S. Nakamura, N. Shibata, Angew. Chem. 2010, 122, 582–586;
10.1002/ange.200905225 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 572–576;
- 9gH. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Angew. Chem. 2011, 123, 7949–7952; Angew. Chem. Int. Ed. 2011, 50, 7803–7806.
- 10
- 10aK. Matoba, H. Kawai, T. Furukawa, A. Kusuda, E. Tokunaga, S. Nakamura, M. Shiro, N. Shibata, Angew. Chem. 2010, 122, 5898–5902;
10.1002/ange.201002065 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5762–5766;
- 10bH. Kawai, Y. Sugita, E. Tokunaga, H. Sato, M. Shiro, N. Shibata, Chem. Commun. 2012, 48, 3632–3634;
- 10cH. Kawai, T. Kitayama, E. Tokunaga, T. Matsumoto, H. Sato, M. Shiro, N. Shibata, Chem. Commun. 2012, 48, 4067–4069;
- 10dH. Kawai, S. Okusu, E. Tokunaga, H. Sato, M. Shiro, N. Shibata, Angew. Chem. 2012, 124, 5043–5046; Angew. Chem. Int. Ed. 2012, 51, 4959–4962.
- 11
- 11aH. Aebi, B. Dewald, H. Suter, Helv. Chim. Acta 1965, 48, 656–674;
- 11bE. G. Samsel, J. K. Kochi, Inorg. Chem. 1986, 25, 2450–2451;
- 11cE. Kosower, Acc. Chem. Res. 1971, 4, 193–198;
- 11dT. Taniguchi, Y. Sugiura, H. Zaimoku, H. Ishibashi, Angew. Chem. 2010, 122, 10352–10355; Angew. Chem. Int. Ed. 2010, 49, 10154–10157.
- 12We sincerely acknowledge one of the reviewers on our manuscript for his/her smart suggestion concerning mechanism of our oxidation system.
- 13The base is indispensable for this oxidation since no reaction was observed in the absence of base (entry 3, Table 1).
- 14Although a catalytic amount of base is enough for this oxidation (entry 24, Table 1), we used a stoichiometric amount of base because of the shorter reaction time and higher chemical yields. This could be explained by a poor solubility of base in organic solvent.
- 15It should be noted that this hydrazine-induced aerobic epoxidation is not only applicable to trifluoromethyl-disubstituted enones, but also to nonfluorinated disubstituted enones, that is, aerobic epoxidation of the nonfluorinated analogue of 1 a, (Z)-1,3-diphenylbut-2-en-1-one, in the presence of 4 a (5 mol %), H2NNHMe (3.0 equiv), and Cs2CO3 (3.0 equiv) in MTBE gave the nonfluorinated analogue of 3 a, ((2S,3R)-3-methyl-3-phenyloxiran-2-yl)(phenyl)methanone, in 71 % yield as a major isomer (d.r.=73:27), though no enantioselectivity was observed (6 % ee).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.