Synthesis of Single-Chain Sugar Arrays†
Nathalie Baradel
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Search for more papers by this authorDr. Sébastien Fort
Oligosaccharides Chemistry and Biotechnology, Centre de Recherches sur les Macromolécules Végétales (CERMAV-CNRS), BP 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Sami Halila
Oligosaccharides Chemistry and Biotechnology, Centre de Recherches sur les Macromolécules Végétales (CERMAV-CNRS), BP 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Nezha Badi
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Search for more papers by this authorCorresponding Author
Dr. Jean-François Lutz
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)===Search for more papers by this authorNathalie Baradel
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Search for more papers by this authorDr. Sébastien Fort
Oligosaccharides Chemistry and Biotechnology, Centre de Recherches sur les Macromolécules Végétales (CERMAV-CNRS), BP 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Sami Halila
Oligosaccharides Chemistry and Biotechnology, Centre de Recherches sur les Macromolécules Végétales (CERMAV-CNRS), BP 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Nezha Badi
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Search for more papers by this authorCorresponding Author
Dr. Jean-François Lutz
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)
Precision Macromolecular Chemistry, Institut Charles Sadron, UPR22-CNRS, 23 rue du Loess, BP84047, 67034 Strasbourg Cedex 2 (France)===Search for more papers by this authorThe research leading to these results was partially funded by the European Research Council under the Seventh Framework Program of the European Union (FP7/2007-2013) and the ERC grant agreement No. 258593. The CNRS, the University of Strasbourg, and the icFRC are also acknowledged for financial support. We thank M. Legros, O. Gavat, and C. Foussat for the SEC measurements. P. Chaud and S. Pradeau are also acknowledged for their help in the preparation of azido sugars.
Graphical Abstract
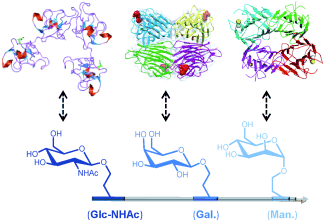
Hexosen in Reih und Glied: Wohldefinierte lineare Polystyrolketten mit präzise positionierten Hexosegruppen (Mannose, Galactose und N-Acetylglucosamin) wurden durch sequenzkontrollierte Polymerisation synthetisiert und in ortsselektiven Modifizierungsschritten weiter umgesetzt. Die multifunktionellen Hexose-Reihen assoziieren mit komplementären Lectinen (siehe Bild).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209052_sm_miscellaneous_information.pdf630.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Ouchi, N. Badi, J.-F. Lutz, M. Sawamoto, Nat. Chem. 2011, 3, 917.
- 2
- 2aH. F. Gao, Macromol. Rapid Commun. 2012, 33, 722;
- 2bJ. Xu, S. Z. Luo, W. F. Shi, S. Y. Liu, Langmuir 2006, 22, 989;
- 2cX. C. Pang, L. Zhao, M. Akinc, J. K. Kim, Z. Q. Lin, Macromolecules 2011, 44, 3746;
- 2dO. Altintas, C. Barner-Kowollik, Macromol. Rapid Commun. 2012, 33, 958;
- 2eE. Harth, B. Van Horn, V. Y. Lee, D. S. Germack, C. P. Gonzales, R. D. Miller, C. J. Hawker, J. Am. Chem. Soc. 2002, 124, 8653.
- 3
- 3aE. J. Foster, E. B. Berda, E. W. Meijer, J. Am. Chem. Soc. 2009, 131, 6964;
- 3bE. B. Berda, E. J. Foster, E. W. Meijer, Macromolecules 2010, 43, 1430;
- 3cE. J. Foster, E. B. Berda, E. W. Meijer, J. Polym. Sci. Part A 2011, 49, 118;
- 3dT. Mes, R. van der Weegen, A. R. A. Palmans, E. W. Meijer, Angew. Chem. 2011, 123, 5191;
10.1002/ange.201100104 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5085.
- 4
- 4aT. Terashima, T. Mes, T. F. A. De Greef, M. A. J. Gillissen, P. Besenius, A. R. A. Palmans, E. W. Meijer, J. Am. Chem. Soc. 2011, 133, 4742;
- 4bN. Giuseppone, J.-F. Lutz, Nature 2011, 473, 40.
- 5
- 5aN. Badi, J.-F. Lutz, Chem. Soc. Rev. 2009, 38, 3383;
- 5bJ.-F. Lutz, Polym. Chem. 2010, 1, 55.
- 6
- 6aS. Pfeifer, J.-F. Lutz, J. Am. Chem. Soc. 2007, 129, 9542;
- 6bS. Pfeifer, J.-F. Lutz, Chem. Eur. J. 2008, 14, 10949;
- 6cL. Hartmann, H. G. Börner, Adv. Mater. 2009, 21, 3425;
- 6dS. Ida, T. Terashima, M. Ouchi, M. Sawamoto, J. Am. Chem. Soc. 2009, 131, 10808;
- 6eM. L. McKee, P. J. Milnes, J. Bath, E. Stulz, A. J. Turberfield, R. K. O’Reilly, Angew. Chem. 2010, 122, 8120;
10.1002/ange.201002721 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7948;
- 6fK. Satoh, S. Ozawa, M. Mizutani, K. Nagai, M. Kamigaito, Nat. Commun. 2010, 1, 1;
- 6gY. Hibi, M. Ouchi, M. Sawamoto, Angew. Chem. 2011, 123, 7572;
10.1002/ange.201103007 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7434.
- 7J.-F. Lutz, B. V. K. J. Schmidt, S. Pfeifer, Macromol. Rapid Commun. 2011, 32, 127.
- 8
- 8aM. Zamfir, J.-F. Lutz, Nat. Commun. 2012, 3, 1138;
- 8bR. Kakuchi, M. Zamfir, J.-F. Lutz, P. Theato, Macromol. Rapid Commun. 2012, 33, 54;
- 8cS. Srichan, D. Chan-Seng, J.-F. Lutz, ACS Macro Lett. 2012, 1, 589;
- 8dS. Srichan, L. Oswald, M. Zamfir, J.-F. Lutz, Chem. Commun. 2012, 48, 1517.
- 9D. Chan-Seng, M. Zamfir, J.-F. Lutz, Angew. Chem. 2012, 124, 12420;
10.1002/ange.201206371 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12254.
- 10
- 10aB. V. K. J. Schmidt, N. Fechler, J. Falkenhagen, J.-F. Lutz, Nat. Chem. 2011, 3, 234;
- 10bM. A. Berthet, Z. Zarafshani, S. Pfeifer, J.-F. Lutz, Macromolecules 2010, 43, 44;
- 10cM. Zamfir, P. Theato, J.-F. Lutz, Polym. Chem. 2012, 3, 1796.
- 11
- 11aC. R. Becer, Macromol. Rapid Commun. 2012, 33, 742;
- 11bV. Ladmiral, E. Melia, D. M. Haddleton, Eur. Polym. J. 2004, 40, 431;
- 11cV. Ladmiral, G. Mantovani, G. J. Clarkson, S. Cauet, J. L. Irwin, D. M. Haddleton, J. Am. Chem. Soc. 2006, 128, 4823;
- 11dS. Muthukrishnan, M. Zhang, M. Burkhardt, M. Drechsler, H. Mori, A. H. E. Müller, Macromolecules 2005, 38, 7926;
- 11eK. Sasaki, Y. Nishida, T. Tsurumi, H. Uzawa, H. Kondo, K. Kobayashi, Angew. Chem. 2002, 114, 4643;
Angew. Chem. Int. Ed. 2002, 41, 4463.
10.1002/1521-3773(20021202)41:23<4463::AID-ANIE4463>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 12
- 12aH. Kunz, Angew. Chem. 1987, 99, 297; Angew. Chem. Int. Ed. Engl. 1987, 26, 294;
- 12bS. J. Danishefsky, M. T. Bilodeau, Angew. Chem. 1996, 108, 1482;
10.1002/ange.19961081304 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1380;
- 12cK. C. Nicolaou, C. N. C. Boddy, S. Bräse, N. Winssinger, Angew. Chem. 1999, 111, 2230;
10.1002/(SICI)1521-3757(19990802)111:15<2230::AID-ANGE2230>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2096.10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 13D. Ponader, F. Wojcik, F. Beceren-Braun, J. Dernedde, L. Hartmann, Biomacromolecules 2012, 13, 1845.
- 14F. Fazio, M. C. Bryan, O. Blixt, J. C. Paulson, C.-H. Wong, J. Am. Chem. Soc. 2002, 124, 14397.
- 15
- 15aV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596;10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 15bM. Meldal, C. W. Tornoe, Chem. Rev. 2008, 108, 2952;
- 15cJ.-F. Lutz, Angew. Chem. 2007, 119, 1036;
10.1002/ange.200604050 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1018.
- 16
- 16aV. Hong, S. I. Presolski, C. Ma, M. G. Finn, Angew. Chem. 2009, 121, 10063; Angew. Chem. Int. Ed. 2009, 48, 9879;
- 16bA. J. T. Dirks, S. S. van Berkel, N. S. Hatzakis, J. A. Opsteen, F. L. van Delft, J. Cornelissen, A. E. Rowan, J. C. M. van Hest, F. Rutjes, R. J. M. Nolte, Chem. Commun. 2005, 4172;
- 16cB. Le Droumaguet, K. Velonia, Macromol. Rapid Commun. 2008, 29, 1073;
- 16dJ.-F. Lutz, H. G. Börner, K. Weichenhan, Aust. J. Chem. 2007, 60, 410.
- 17I. E. Valverde, A. F. Delmas, V. Aucagne, Tetrahedron 2009, 65, 7597.
- 18P. G. M. Wuts, T. W. Greene, Greene’s Protective Groups in Organic Synthesis, 4th ed., Wiley, New York, 2006.
10.1002/0470053488 Google Scholar
- 19Since the polymers contain a small fraction of protected alkynes, errors in the integration of the NMR spectrum are expected. Thus, it cannot be fully excluded that some TES and TIPS groups are cleaved during the steps for the removal of the TMS and TES groups, respectively. However, if they exist, such deviations from ideal orthogonality are beyond the detection limit of the method.
- 20H. Ito, K. Arimoto, H.-o. Sensul, A. Hosomi, Tetrahedron Lett. 1997, 38, 3977.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.