Micelle-like Molecular Capsules with Anthracene Shells as Photoactive Hosts†
Kei Kondo
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorAkira Suzuki
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorProf. Dr. Munetaka Akita
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Michito Yoshizawa
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)===Search for more papers by this authorKei Kondo
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorAkira Suzuki
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorProf. Dr. Munetaka Akita
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Michito Yoshizawa
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503 (Japan)===Search for more papers by this authorThis research was supported by the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for Next-Generation World-Leading Researchers” and by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) through a Grant-in-Aid for Scientific Research on Innovative Areas (“Coordination Programming”). We thank K. Yoza (Bruker AXS) for supporting X-ray crystallographic analysis, S. Moriguchi and A. Kogure (Shimadzu Analytical & Measuring Center, Inc.) for AFM measurement, and M. Maruyama (Shimadzu Corp.) and Y. Uratani (Beckman Coulter K.K.) for particle-size analysis. We also thank Dr. J. Klosterman (BGSU) for helpful discussions.
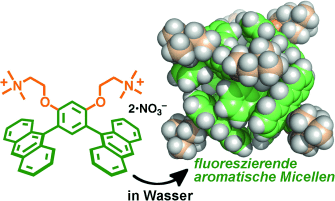
Graphical Abstract
Aromatische Micellen: Hydrophobe und Aren-Aren-Wechselwirkungen vermitteln die spontane Bildung micellartiger molekularer Kapseln mit großen aromatischen Schalen aus gebogenen Bisanthracen-Amphiphilen (siehe Bild). Die micellaren Kapseln können in Wasser Fluoreszenzfarbstoffe aufnehmen, und die resultierenden Nanokomposite zeigen starke Fluoreszenz aufgrund eines effizienten Energietransfers von der Schale zum verkapselten Gastmolekül.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201208643_sm_miscellaneous_information.pdf5.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Moroi, Micelles: Theoretical and Applied Aspects, Plenum, New York, 1992.
10.1007/978-1-4899-0700-4 Google Scholar
- 2D. Myers, Surfactant Science and Technology, 3rd ed., Wiley, Hoboken, 2006.
- 3J. W. Steed, J. L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, Chichester, 2009.
10.1002/9780470740880 Google Scholar
- 4
- 4aD. J. Cram, S. Karbach, Y. H. Kim, L. Baczynskyj, G. W. Kalleymeyn, J. Am. Chem. Soc. 1985, 107, 2575–2576;
- 4bD. J. Cram, J. M. Cram, Container Molecules and Their Guests, Royal Society of Chemistry, Cambridge, 1994.
- 5R. S. Meissner, J. de Mendoza, J. Rebek, Jr., Science 1995, 270, 1485–1488.
- 6L. R. MacGillivray, J. L. Atwood, Nature 1997, 389, 469–472.
- 7D. L. Caulder, R. E. Powers, T. N. Parac, K. N. Raymond, Angew. Chem. 1998, 110, 1940–1943;
10.1002/(SICI)1521-3757(19980703)110:13/14<1940::AID-ANGE1940>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1840–1843.10.1002/(SICI)1521-3773(19980803)37:13/14<1840::AID-ANIE1840>3.0.CO;2-D CAS Web of Science® Google Scholar
- 8M. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi, K. Ogura, Nature 1995, 378, 469–471.
- 9P. Mal, B. Breiner, K. Rissanen, J. R. Nitschke, Science 2009, 324, 1697–1699.
- 10J. Kang, J. Rebek, Jr., Nature 1997, 385, 50–52.
- 11M. Yoshizawa, M. Tamura, M. Fujita, Science 2006, 312, 251–254.
- 12M. D. Pluth, R. G. Bergman, K. N. Raymond, Science 2007, 316, 85–88.
- 13M. D. Watson, A. Fechtenkötter, K. Müllen, Chem. Rev. 2001, 101, 1267–1300.
- 14R. J. Bushby, O. R. Lozman, Curr. Opin. Colloid Interface Sci. 2002, 7, 343–354.
- 15
- 15aN. Takeda, K. Umemoto, K. Yamaguchi, M. Fujita, Nature 1999, 398, 794–796;
- 15bS. Hiraoka, K. Harano, M. Shiro, Y. Ozawa, N, Yasuda, K. Toriumi, M. Shionoya, Angew. Chem. 2006, 118, 6638–6641;
10.1002/ange.200601431 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6488–6491.
- 16C. L. D. Gibb, B. C. Gibb, J. Am. Chem. Soc. 2004, 126, 11408–11409.
- 17S. Hiraoka, K. Harano, M. Shiro, M. Shionoya, J. Am. Chem. Soc. 2008, 130, 14368–14369.
- 18
- 18aS. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev. 2000, 100, 853–908;
- 18bF. Hof, S. L. Craig, C. Nuckolls, J. Rebek, Jr., Angew. Chem. 2002, 114, 1556–1578;
10.1002/1521-3757(20020503)114:9<1556::AID-ANGE1556>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1488–1508;10.1002/1521-3773(20020503)41:9<1488::AID-ANIE1488>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 18cJ. Rebek, Jr. Angew. Chem. 2005, 117, 2104–2115;
10.1002/ange.200462839 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2068–2078;
- 18dD. M. Vriezema, M. C. Aragonès, J. Elemans, J. Cornelissen, A. E. Rowan, R. J. M. Nolte, Chem. Rev. 2005, 105, 1445–1489;
- 18eM. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. 2009, 121, 3470–3490; Angew. Chem. Int. Ed. 2009, 48, 3418–3438;
- 18fH. Amouri, C. Desmarets, J. Moussa, Chem. Rev. 2012, 112, 2015–2041.
- 19Although the encapsulation of fluorescent molecules by self-assembled hosts has been reported, enhanced fluorescence from the encapsulated guest molecules through host–guest energy transfer has seldom been observed:
- 19aS. J. Dalgarno, S. A. Tucker, D. B. Bassil, J. L. Atwood, Science 2005, 309, 2037–2039;
- 19bL. S. Kaanumalle, C. L. D. Gibb, B. C. Gibb, V. Ramamurthy, J. Am. Chem. Soc. 2005, 127, 3674–3675;
- 19cK. Ono, J. K. Klosterman, M. Yoshizawa, K. Sekiguchi, T. Tahara, M. Fujita, J. Am. Chem. Soc. 2009, 131, 12526–12527;
- 19dN. Nishimura, K. Kobayashi, J. Org. Chem. 2010, 75, 6079–6085.
- 20For efficient FRET emission from guest chromophores in organogels, see:
- 20aA. Ajayaghosh, S. J. George, V. K. Praveen, Angew. Chem. 2003, 115, 346–349;
10.1002/ange.200390077 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 332–335;
- 20bA. Ajayaghosh, C. Vijayakumar, V. K. Praveen, S. S. Babu, R. Varghese, J. Am. Chem. Soc. 2006, 128, 7174–7175;
- 20cA. Ajayaghosh, V. K. Praveen, C. Vijayakumar, S. J. George, Angew. Chem. 2007, 119, 6376–6381;
10.1002/ange.200701925 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6260–6265.
- 21
- 21aN. Kishi, Z. Li, K. Yoza, M. Akita, M. Yoshizawa, J. Am. Chem. Soc. 2011, 133, 11438–11441;
- 21bZ. Li, N. Kishi, K. Hasegawa, M. Akita, M. Yoshizawa, Chem. Commun. 2011, 47, 8605–8607;
- 21cZ. Li, N. Kishi, K. Yoza, M. Akita, M. Yoshizawa, Chem. Eur. J. 2012, 18, 8358–8365;
- 21dK. Yazaki, N. Kishi, M. Akita, M. Yoshizawa, Chem. Commun. 2013, DOI: 10.1039/c3cc38869g.
- 22K. Hagiwara, Y. Sei, M. Akita, M. Yoshizawa, Chem. Commun. 2012, 48, 7678–7680.
- 23See the Supporting Information. In the NOESY spectrum of 2 a, two sets of correlation signals (He–Hg and Hb–Hd) were observed between different amphiphilic molecules (see Figure S55).
- 24The IG method can be used to determine a particle size of less than 10 nm through measurement of the diffusion coefficient from the decay rate of the diffracted light intensity in the relaxation process of particle-density modulation by dielectrophoresis: Y. Wada, S. Totoki, M. Watanabe, N. Moriya, Y. Tsunazawa, H. Shimaoka, Opt. Express 2006, 14, 5755–5764.
- 25S. Honda, T. Yamamoto, Y. Tezuka, J. Am. Chem. Soc. 2010, 132, 10251–10253.
- 26The emission lifetime (τ) of 2 a (23 ns) is longer than that of 1 a (11 ns).
- 271H NMR spectra of 2 a⊃3 and 2 a⊃4 in D2O revealed that the aromatic signals from the shells of 2 a were hardly altered by the encapsulation of guest 3 or 4. This result indicates that the shape and size of the capsule framework are retained, as also evidenced by DOSY NMR spectroscopic analysis (see Figure S44 and S46). In contrast, the proton signals of the encapsulated guests were significantly broadened owing to restriction of the molecular motion by the limited cavity of 2 a (see Figures S42 and S45). Thus, the NOESY and DOSY spectra exhibited only signals derived from the host framework. When CD3OD (20–80 % v/v) was added to solutions of the host–guest complexes in D2O, the guest signals in the 1H NMR spectra were observed with large upfield shifts (see Figures S53 and S54).
- 28The absorption spectrum of 2 a⊃4 in methanol revealed that the ratio of 1 a to 4 is 20:1, which indicates that approximately 20 % of capsule 2 a binds one molecule of 4. The increase in solvent polarity upon the addition of the salt NaNO3 promotes the enclathration of dye 4 by capsule 2 a.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.