An Asymmetric Hydroformylation Catalyst that Delivers Branched Aldehydes from Alkyl Alkenes†
Dr. Gary M. Noonan
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorDr. José A. Fuentes
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorCorresponding Author
Dr. Christopher J. Cobley
Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Christopher J. Cobley, Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Matthew L. Clarke, School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorCorresponding Author
Dr. Matthew L. Clarke
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Christopher J. Cobley, Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Matthew L. Clarke, School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorDr. Gary M. Noonan
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorDr. José A. Fuentes
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorCorresponding Author
Dr. Christopher J. Cobley
Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Christopher J. Cobley, Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Matthew L. Clarke, School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorCorresponding Author
Dr. Matthew L. Clarke
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Christopher J. Cobley, Chirotech Technology Ltd., Dr. Reddy's Laboratories (EU) Limited, 410 Cambridge Science Park, Milton Road, Cambridge, CB4 OPE (UK)
Matthew L. Clarke, School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, KY16 9ST (UK)
Search for more papers by this authorThe authors thank the EPSRC and Dr. Reddy’s Laboratories for funding, and EPSRC for the use of the National Mass Spectrometry Service.
Graphical Abstract
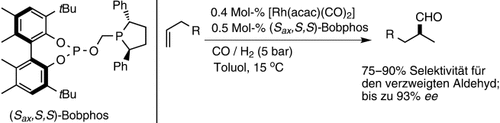
Überraschende Selektivität: Mithilfe des neuen chiralen Liganden Bobphos gelangen die ersten enantioselektiven Hydroformylierungen einfacher Alkene des Typs RCH2CHCH2, die bevorzugt den verzweigten Aldehyd liefern (siehe Schema). Etablierte Liganden sind in dieser Reaktion unselektiv oder zeigen eine leichte Bevorzugung des linearen Produkts.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201108203_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. W. N. M. Van Leeuwen, C. Claver, Rhodium Catalysed Hydroformylation, Kluwer, Dordrecht, 2000;
- 1bF. Ungváry, Coord. Chem. Rev. 2004, 248, 867;
- 1cF. Agbossou, J. F. Carpentier, A. Mortreaux, Chem. Rev. 1995, 95, 2485;
- 1dM. Diéguez, O. Pàmies, C. Claver, Tetrahedron: Asymmetry 2004, 15, 2113.
- 2
- 2aM. Diéguez, O. Pàmies, A. Ruiz, S. Castillón, C. Claver, Chem. Eur. J. 2001, 7, 3086;
10.1002/1521-3765(20010716)7:14<3086::AID-CHEM3086>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 2bA. Gual, C. Godard, S. Castillón, C. Claver, Adv. Synth. Catal. 2010, 352, 463;
- 2cC. J. Cobley, K. Gardner, J. Klosin, C. Praquin, C. Hill, G. T. Whiteker, A. Zanotti-Gerosa, J. L. Peterson, K. A. Abboud, J. Org. Chem. 2004, 69, 4031;
- 2dC. J. Cobley, J. Klosin, C. Qin, G. T. Whiteker, Org. Lett. 2004, 6, 3277;
- 2eS. Breeden, D. J. Cole-Hamilton, D. F. Foster, G. J. Schwarz, M. Wills, Angew. Chem. 2000, 112, 4272;
10.1002/1521-3757(20001117)112:22<4272::AID-ANGE4272>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4106;10.1002/1521-3773(20001117)39:22<4106::AID-ANIE4106>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 2fJ. E. Babin, G. T. Whiteker, 1993, WO93/03839;
- 2gK. Nozaki, N. Sakai, T. Nanno, T. Higashijima, S. Mano, T. Horiuchi, H. Takaya, J. Am. Chem. Soc. 1997, 119, 4413;
- 2hY. Yan, X. Zhang, J. Am. Chem. Soc. 2006, 128, 7198;
- 2iT. P. Clark, C. R. Landis, S. L. Feed, J. Klosin, K. A. Abboud, J. Am. Chem. Soc. 2005, 127, 5040;
- 2jA. T. Axtell, C. J. Cobley, J. Klosin, G. T. Whiteker, A. Zanotti-Gerosa, K. A. Abboud, Angew. Chem. 2005, 117, 5984;
10.1002/ange.200501478 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5834;
- 2kF. Doro, J. N. H. Reek, P. W. N. M. van Leeuwen, Organometallics 2010, 29, 4440;
- 2lM. Rubio, A. Suárez, E. Álvarez, C. Bianchini, W. Oberhauser, M. Peruzzini, A. Pizzano, Organometallics 2007, 26, 6428;
- 2mS. Deerenberg, P. C. J. Kamer, P. W. N. M. Van Leeuwen, Organometallics 2000, 19, 2065;
- 2nA. L. Watkins, B. G. Hashiguchi, C. R. Landis, Org. Lett. 2008, 10, 4553;
- 2oJ. Wassenaar, B. de Bruin, J. N. H. Reek, Organometallics 2010, 29, 2767;
- 2pC. G. Arena, F. Faraone, C. Graiff, A. Tiripicchio, Eur. J. Inorg. Chem. 2002, 711;
- 2qO. Pàmies, G. Net, A. Ruiz, C. Claver, Tetrahedron: Asymmetry 2002, 12, 3441;
- 2rR. Ewalds, E. B. Eggeling, A. C. Hewat, P. C. J. Kamer, O. W. N. M. Van Leeuwen, D. Vogt, Chem. Eur. J. 2000, 6, 1496.
10.1002/(SICI)1521-3765(20000417)6:8<1496::AID-CHEM1496>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 3
- 3aM. L. Clarke, Curr. Org. Chem. 2005, 9, 701;
- 3bB. Breit, W. Seiche, Synthesis 2001, 1; selected examples of the synthesis of functionalized aldehydes:
- 3cM. L. Clarke, G. J. Roff, Chem. Eur. J. 2006, 12, 7978;
- 3dO. Abillard, B. Breit, Adv. Synth. Catal. 2007, 349, 1891;
- 3eP. Eilbracht, A. Schmidt, Top. Organomet. Chem. 2006, 18, 65;
- 3fG. M. Noonan, D. Newton, C. J. Cobley, A. Suárez, A. Pizzano, M. L. Clarke, Adv. Synth. Catal. 2010, 352, 1047;
- 3gA. Farwick, G. Helmchen, Adv. Synth. Catal. 2010, 352, 1023;
- 3hB. Breit, D. Breuninger, J. Am. Chem. Soc. 2004, 126, 10244;
- 3iE. Airiau, T. Spangenberg, N. Girard, B. Breit, A. Mann, Org. Lett. 2010, 12, 528;
- 3jC. Botteghi, T. Corrias, M. Marchetti, S. Pagnelli, O. Piccolo, Org. Process Res. Dev. 2002, 6, 379;
- 3kX. Zhang, B. Cao, S. Yu, X. Zhang, Angew. Chem. 2010, 122, 4141; Angew. Chem. Int. Ed. 2010, 49, 4047;
- 3lT. Horiuchi, T. Ohta, E. Shirakawa, K. Nozaki, H. Takaya, J. Org. Chem. 1997, 62, 4285;
- 3mK. Nozaki, W. G. Li, T. Horiuchi, H. Takaya, Tetrahedron Lett. 1997, 38, 4611;
- 3nT. Higashizima, N. Sakai, K. Nozaki, H. Takaya, Tetrahedron Lett. 1994, 35, 2023;
- 3oC. Cobley, G. Meek, C. Rand, Tetrahedron Lett. 2011, 52, 3271;
- 3pR. I. McDonald, G. W. Wong, R. P. Neupane, S. S. Stahl, C. R. Landis, J. Am. Chem. Soc. 2010, 132, 14027;
- 3qG. M. Noonan, C. J. Cobley, T. Lebl, M. L. Clarke, Chem. Eur. J. 2010, 16, 12788;
- 3rX. Wang, S. L. Buchwald, J. Am. Chem. Soc. 2011, 133, 19080.
- 4
- 4aX. Sun, K. Fripong, K. L. Tan, J. Am. Chem. Soc. 2010, 132, 11841;
- 4bA. D. Worthy, C. L. Joe, T. E. Lightburn, K. L. Tan, J. Am. Chem. Soc. 2010, 132, 14757;
- 4cT. E. Lightburn, M. T. Dombrowski, K. L. Tan, J. Am. Chem. Soc. 2008, 130, 9210;
- 4dC. U. Grünanger, B. Breit, Angew. Chem. 2010, 122, 979;
10.1002/ange.200905949 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 967, and references therein.
- 5M. Jackson, I. C. Lennon, Tetrahedron Lett. 2007, 48, 1831.
- 6
- 6aV. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek, J. Am. Chem. Soc. 2004, 126, 1526;
- 6bR. A. Baber, M. L. Clarke, K. Heslop, A. Marr, A. G. Orpen, P. G. Pringle, A. M. Ward, D. A. Zambrano-Williams, Dalton Trans. 2005, 1079;
- 6cA. A. Dabbawala, R. V. Jasra, H. C. Bajaj, Catal. Commun. 2011, 12, 403;
- 6dsee also R. Bellini, S. H. Chikkali, G. Berthon-Gelloz, J. N. H. Reek, Angew. Chem. 2011, 123, 7480;
10.1002/ange.201101653 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7342;
- 6eM. Rosa Axet, S. Castillon, C. Claver, Inorg. Chim. Acta 2006, 359, 2973;
- 6fP. Kalck, D. C. Park, F. Serein, J. Mol. Catal. 1986, 36, 349;
- 6gE. Zuidema, P. Elsbeth Goudriaan, B. H. G. Swennenhuis, P. C. J. Kamer, P. W. N. M. van Leeuwen, M. Lutz, A. L. Spek, Organometallics 2010, 29, 1210.
- 7K. Nozaki, T. Nanno, H. Takaya, J. Organomet. Chem. 1997, 527, 103.
- 8
- 8aA. Lightfoot, P. Schnider, A. Pfaltz, Angew. Chem. 1998, 110, 3047;
10.1002/(SICI)1521-3757(19981016)110:20<3047::AID-ANGE3047>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2897;10.1002/(SICI)1521-3773(19981102)37:20<2897::AID-ANIE2897>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 8bL. Saudan, Acc. Chem. Res. 2007, 40, 1309.
- 9D. J. Ager, S. Babler, D. E. Froen, S. A. Laneman, D. P. Panaleone, I. Prakash, B. Zhi, Org. Process Res. Dev. 2003, 7, 369.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.