Catalytic Asymmetric Intermolecular Stetter Reactions of Enolizable Aldehydes with Nitrostyrenes: Computational Study Provides Insight into the Success of the Catalyst†
Daniel A. DiRocco
Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorElizabeth L. Noey
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. N. Houk
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
K. N. Houk, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Tomislav Rovis, Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorCorresponding Author
Prof. Tomislav Rovis
Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
K. N. Houk, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Tomislav Rovis, Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorDaniel A. DiRocco
Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorElizabeth L. Noey
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. N. Houk
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
K. N. Houk, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Tomislav Rovis, Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorCorresponding Author
Prof. Tomislav Rovis
Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
K. N. Houk, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095 (USA)
Tomislav Rovis, Department of Chemistry, Colorado State University, Fort Collins, CO 80526 (USA)
Search for more papers by this authorWe thank NIGMS for generous support of this research (GM 36700 to K.N.H. and GM 72586 to T.R.). T.R. thanks Amgen and Roche for unrestricted support. E.L.N. is grateful to the National Institutes of Health Chemistry-Biology Interface Training Program Grant (T32M008496). K.N.H. is grateful to the National Science Foundation (CHE-0548209) for financial support, to the UCLA Academic Technology Services (ATS) Hoffman2 and IDRE clusters for computational resources, and for the TeraGrid resources provided by NCSA (CHE-0400414).
Graphical Abstract
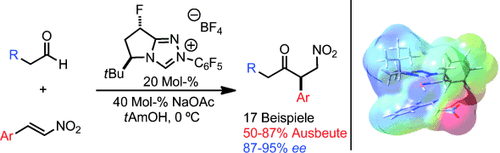
Fluor hilft: Ein fluoriertes Triazoliumsalz wurde entwickelt, das als Präkatalysator für die asymmetrische intermolekulare Stetter-Reaktion von enolisierbaren Aldehyden und Nitrostyrolen dient (siehe Schema). Die trans-Fluorierung der Katalysatorarchitektur führt zu einer unerreichten Reaktivität und Enantioselektivität in der gewünschten Reaktion. Eine DFT-Studie liefert Belege für eine elektrostatische Wechselwirkung als die Quelle der gesteigerten Enantioinduktion.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201107597_sm_miscellaneous_information.pdf5.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aN. Marion, S. Díez-González, S. P. Nolan, Angew. Chem. 2007, 119, 3046–3058;
10.1002/ange.200603380 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2988–3000;
- 1bD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606–5655;
- 1cJ. Moore, T. Rovis, Top. Curr. Chem. 2009, 291, 77–144;
- 1dH. U. Vora, T. Rovis, Aldrichimica Acta 2011, 44, 3–11.
- 2
- 2aH. Stetter, M. Schrecke, Angew. Chem. 1973, 85, 89; Angew. Chem. Int. Ed. Engl. 1973, 12, 81;
- 2bH. Stetter, Angew. Chem. 1976, 88, 695–704; Angew. Chem. Int. Ed. Engl. 1976, 15, 639–647;
- 2cH. Stetter, H. Kuhlmann, Org. React. 1991, 40, 407–496;
- 2dM. Christmann, Angew. Chem. 2005, 117, 2688–2690;
10.1002/ange.200500761 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2632–2634;
- 2eT. Rovis, Chem. Lett. 2008, 37, 2–7.
- 3For a review on the asymmetric intramolecular Stetter reaction, see: J. Read de Alaniz, T. Rovis, Synlett 2009, 1189–1207.
- 4
- 4aQ. Liu, S. Perreault, T. Rovis, J. Am. Chem. Soc. 2008, 130, 14066–14067;
- 4bQ. Liu, T. Rovis, Org. Lett. 2009, 11, 2856–2859;
- 4cD. A. DiRocco, K. M. Oberg, D. M. Dalton, T. Rovis, J. Am. Chem. Soc. 2009, 131, 10872–10874;
- 4dD. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2011, 133, 10402–10405.
- 5For other contributions to the asymmetric intermolecular Stetter reaction, see:
- 5aD. Enders, J. Han, A. Henseler, Chem. Commun. 2008, 3989–3991;
- 5bD. Enders, J. Han, Synthesis 2008, 3864–3868;
- 5cT. Jousseaume, N. E. Wurz, F. Glorius, Angew. Chem. 2011, 123, 1446–1450; Angew. Chem. Int. Ed. 2011, 50, 1410–1414;
- 5dE. Sánchez-Larios, K. Thai, F. Bilodeau, M. Gravel, Org. Lett. 2011, 13, 4942–4945;
- 5eX. Fang, X. Chen, H. Lv, Y. R. Chi, Angew. Chem. 2011, 123, 11986–11989; Angew. Chem. Int. Ed. 2011, 50, 11782–11785.
- 6For reviews on the effect of fluorine on molecular conformation, see:
- 6aL. Hunter, Beilstein J. Org. Chem. 2010, 6, No. 38;
- 6bL. E. Zimmer, C. Sparr, R. Gilmour, Angew. Chem. 2011, 123, 12062–12074;
10.1002/ange.201102027 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11860–11871.
- 7Enders has reported the asymmetric Stetter of butanal and chalcone with two different chiral thiazolium catalysts (4 % yield, 39 % ee and 29 % yield, 30 % ee); see:
- 7aD. Enders, B. Bockstiegel, H. Dyker, U. Jegelka, H. Kipphardt, D. Kownatka, H. Kuhlmann, D. Mannes, J. Tiebes, K. Papadopoulos in “Wege zu neuen Verfahren und Produkten der Biotechnologie”, DECHEMA-Monographie 1993, 129, 209;
- 7bD. Enders in Stereoselective Synthesis (Eds.: ), Springer, Berlin, 1994, p. 63;
10.1007/978-3-642-78496-5_4 Google Scholar
- 7cD. Enders, K. Breuer, Comprehensive Asymmetric Catalysis, Springer, Berlin, 1999, pp. 1093–1104;
10.1007/978-3-642-58571-5_4 Google Scholar
- 7dD. Enders, T. Balensiefer, Acc. Chem. Res. 2004, 37, 534–541.
- 8
- 8aGlorius et al. reported a single example of an asymmetric intermolecular Stetter reaction of an aliphatic aldehyde adding to a β-unsubstituted Michael acceptor; see ref. [5c];
- 8bAn enzyme catalyzed asymmetric Stetter reaction has recently been published, providing Stetter products of acetaldehyde: C. Dresen, M. Richter, M. Pohl, S. Lüdeke, M. Müller, Angew. Chem. 2010, 122, 6750–6753;
10.1002/ange.201000632 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6600–6603.
- 9The use of stronger bases and/or more polar solvents leads to base-induced decomposition products.
- 10See the Supporting Information for complete experimental details.
- 11J. M. Um, D. A. DiRocco, E. L. Noey, T. Rovis, K. N. Houk, J. Am. Chem. Soc. 2011, 133, 11249–11254.
- 12M. J. Frisch, et al. Gaussian 09, revision A.1, Gaussian, Inc., Wallingford, CT, 2009 (See the Supporting Information for full reference).
- 13
- 13aV. Barone, M. Cossi, J. Phys. Chem. A 1998, 102, 1995–2001;
- 13bM. Cossi, N. Rega, G. Scalmani, V. Barone, J. Comput. Chem. 2003, 24, 669–681.
- 14L. Simón, J. M. Goodman, Org. Biomol. Chem. 2011, 9, 689–700.
- 15
- 15aT. Dudding, K. N. Houk, Proc. Natl. Acad. Sci. USA 2004, 101, 5770–5775;
- 15bK. J. Hawkes, B. F. Yates, Eur. J. Org. Chem. 2008, 5563–5570.
- 16
- 16aY. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241;
- 16bY. Zhao, D. G. Truhlar, Acc. Chem. Res. 2008, 41, 157–167.
- 17J. L. Moore, A. P. Silvestri, J. Read de Alaniz, D. A. DiRocco, T. Rovis, Org. Lett. 2011, 13, 1742–1745.
- 18D. Seebach, J. Golinski, Helv. Chim. Acta 1981, 64, 1413–1423.
- 19The anti conformation is favored for TS4 and TS6. The gauche (+) conformation is only slightly favored (0.5 kcal) over the anti conformation for TS2.
- 20The addition of the acyl anion equivalent to the olefin is a concerted reaction reminiscent of the reverse Cope elimination, as we and others have previously proposed; see:
- 20aJ. Read de Alaniz, T. Rovis, J. Am. Chem. Soc. 2005, 127, 6284–6289;
- 20bI. Piel, M. Steinmetz, K. Hirano, R. Fröhlich, S. Grimme, F. Glorius, Angew. Chem. 2011, 123, 5087–5091;
10.1002/ange.201008081 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4983–4987;
- 20cD. A. DiRocco, T. Rovis, Angew. Chem. 2011, 123, 8130–8132;
10.1002/ange.201102920 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7982–7983.
- 21A table of all transition structure energies is given in the Supporting Information.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.