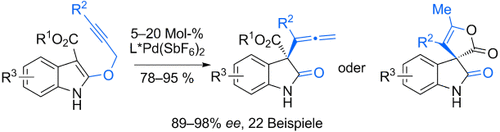
Asymmetric Synthesis of Allenyl Oxindoles and Spirooxindoles by a Catalytic Enantioselective Saucy–Marbet Claisen Rearrangement†
Trung Cao
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorJoshua Deitch
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorDr. Elizabeth C. Linton
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marisa C. Kozlowski
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)Search for more papers by this authorTrung Cao
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorJoshua Deitch
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorDr. Elizabeth C. Linton
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marisa C. Kozlowski
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)
Department of Chemistry, Penn Merck High Throughput Experimentation Laboratory, University of Pennsylvania, Philadelphia, PA 19104-6323 (USA)Search for more papers by this authorFinancial support was provided by the NSF (CHE-0911713) and NIH (RO1GM087605). Partial instrumentation support was provided by the NIH for MS (1S10RR023444) and NMR (1S10RR022442) and by the NSF for an X-ray diffractometer (CHE-0840438). The invaluable assistance of Dr. Patrick Carroll in obtaining the crystal structures is gratefully acknowledged.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201107417_sm_miscellaneous_information.pdf13.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For general reviews, see:
- 1aD. Enders, M. Knopp, R. Schiffers, Tetrahedron: Asymmetry 1996, 7, 1847–1882;
- 1bA. M. M. Castro, Chem. Rev. 2004, 104, 2939–3002;
- 1cK. C. Majumdar, S. Alam, B. Chattopadhyay, Tetrahedron 2008, 64, 597–643; for reviews on asymmetric Claisen rearrangements, see:
- 1dH. Ito, T. Taguchi, Chem. Soc. Rev. 1999, 28, 43–50;
- 1eM. Hiersemann, L. Abraham, Eur. J. Org. Chem. 2002, 1461–1471;
- 1fU. Nubbemeyer, Synthesis 2003, 961–1008;
- 1g The Claisen Rearrangment (Eds.: ), Wiley-VCH, Weinheim, 2007.
- 2
- 2aL. Abraham, R. Czerwonka, M. Hiersemann, Angew. Chem. 2001, 113, 4835–4837;
10.1002/1521-3757(20011217)113:24<4835::AID-ANGE4835>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4700–4703;10.1002/1521-3773(20011217)40:24<4700::AID-ANIE4700>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 2bH. Helmboldt, M. Hiersemann, Tetrahedron 2003, 59, 4031–4038;
- 2cL. Abraham, M. Koerner, M. Hiersemann, Tetrahedron Lett. 2004, 45, 3647–3650;
- 2dL. Abraham, M. Koerner, P. Schwab, M. Hiersemann, Adv. Synth. Catal. 2004, 346, 1281–1294, and references therein.
- 3E. C. Linton, M. C. Kozlowski, J. Am. Chem. Soc. 2008, 130, 16162–16163, and references therein.
- 4
- 4aC. Uyeda, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 9228–9229;
- 4bC. Uyeda, E. N. Jacobsen, J. Am. Chem. Soc. 2011, 133, 5062–5075;
- 4cC. Uyeda, A. R. Rötheli, E. N. Jacobsen, Angew. Chem. 2010, 122, 9947–9950;
10.1002/ange.201005183 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9753–9756.
- 5J. Kaeobamrung, J. Mahatthananchai, P. Zheng, J. W. Bode, J. Am. Chem. Soc. 2010, 132, 8810–8812.
- 6The involvement of a discrete allyl cation gives rise to both [3,3′] and [1,3′] products: M. E. Geherty, R. D. Dura, S. G. Nelson, J. Am. Chem. Soc. 2010, 132, 11875–11877.
- 7
- 7aJ. A. Mulder, R. P. Hsung, M. O. Frederick, M. R. Tracey, C. A. Zificsak, Org. Lett. 2002, 4, 1383–1386;
- 7bM. O. Frederick, R. P. Hsung, R. H. Lambeth, J. A. Mulder, M. R. Tracey, Org. Lett. 2003, 5, 2663–2666;
- 7cK. C. M. Kurtz, M. O. Frederick, R. H. Lambeth, J. A. Mulder, M. R. Tracey, R. P. Hsung, Tetrahedron 2006, 62, 3928–3938;
- 7dY. Tang, L. C. Shen, B. J. Dellaria, R. P. Hsung, Tetrahedron Lett. 2008, 49, 6404–6409.
- 8S. N. Gradl, D. Trauner in The Claisen Rearrangement (Eds.: ), Wiley-VCH, Weinheim, 2007, pp. 367–396.
10.1002/9783527610549.ch7 Google Scholar
- 9K. I. Booker-Milburn, M. Fedouloff, S. J. Paknoham, J. B. Strachan, J. L. Melville, M. Voyle, Tetrahedron Lett. 2000, 41, 4657–4661.
- 10For selected natural products, see:
- 10aS. Takano, K. Ogasawara, Alkaloids 1989, 36, 225–251;
- 10bT. Hino, M. Nakagawa, Alkaloids 1989, 34, 1–75;
- 10cH. J. Wang, J. B. Gloer, D. T. Wicklow, P. F. Dowd, J. Nat. Prod. 1998, 61, 804–807; Notoamides;
- 10dH. Kato, T. Yoshida, T. Tokue, Y. Nojiri, H. Hirota, T. Ohta, R. M. Williams, S. Tsukamoto, Angew. Chem. 2007, 119, 2304–2306; Angew. Chem. Int. Ed. 2007, 46, 2254–2256.
- 11For selected biological agents, see:
- 11aK. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps, S. Wang, J. Am. Chem. Soc. 2005, 127, 10130–10131;
- 11bK. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller, S. Wang, J. Med. Chem. 2006, 49, 3432–3435;
- 11cA. K. Franz, P. D. Dreyfuss, S. L. Schreiber, J. Am. Chem. Soc. 2007, 129, 1020–1021.
- 12For reviews, see: F. Zhou, Y. L. Liu, J. A. Zhou, Adv. Synth. Catal. 2010, 352, 1381–1407.
- 13For selected catalytic asymmetric reactions to access oxindoles with C3 quaternary centers, see:
- 13aS. Ma, X. Han, S. Krishnan, S. C. Virgil, B. M. Stoltz, Angew. Chem. 2009, 121, 8181–8185; Angew. Chem. Int. Ed. 2009, 48, 8037–8041, and references therein;
- 13bY. Kato, M. Furutachi, Z. Chen, H. Mitsunuma, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 9168–9169;
- 13cA. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900–9901;
- 13dT. Bui, S. Syed, C. F. Barbas, J. Am. Chem. Soc. 2009, 131, 8758–8759;
- 13eX. Luan, L. Wu, E. Drinkel, R. Mariz, M. Gatti, R. Dorta, Org. Lett. 2010, 12, 1912–1915;
- 13fX. Li, B. Zhang, Z. G. Xi, S. Luo, J. P. Cheng, Adv. Synth. Catal. 2010, 352, 416–424.
- 14See the Supporting Information.
- 15G. R. Dick, D. M. Knapp, E. P. Gillis, M. D. Burke, Org. Lett. 2010, 12, 2314–2317.
- 16A. Fensome, et al., J. Med. Chem. 2008, 51, 1861–1873.
- 17For reviews, see:
- 17aB. M. Trost, M. K. Brennan, Synthesis 2009, 3003–3025;
- 17bC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902–8912;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748–8758;
- 17cC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209–2219.
- 18For selected asymmetric reactions to access spiroindoles, see:
- 18aX. H. Chen, Q. Wei, S. W. Luo, H. Xiao, L. Z. Gong, J. Am. Chem. Soc. 2009, 131, 13819–13825, and references therein;
- 18bZ. J. Jia, H. Jiang, J. L. Li, B. Gschwend, Q. Z. Li, X. A. Yin, J. Grouleff, Y. C. Chen, K. A. Jorgensen, J. Am. Chem. Soc. 2011, 133, 5053–5061;
- 18cB. Tan, N. R. Candeias, C. F. Barbas, J. Am. Chem. Soc. 2011, 133, 4672–4675;
- 18dX. X. Jiang, Y. M. Cao, Y. Q. Wang, L. P. Liu, F. F. Shen, R. Wang, J. Am. Chem. Soc. 2010, 132, 15328–15333;
- 18eQ. Wei, L. Z. Gong, Org. Lett. 2010, 12, 1008–1011;
- 18fK. Jiang, Z. J. Jia, S. Chen, L. Wu, Y. C. Chen, Chem. Eur. J. 2010, 16, 2852–2856;
- 18gG. Bencivenni, L. Y. Wu, A. Mazzanti, B. Giannichi, F. Pesciaioli, M. P. Song, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 7336–7339;
10.1002/ange.200903192 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7200–7203;
- 18hT. Bui, S. Syed, C. F. Barbas, J. Am. Chem. Soc. 2009, 131, 8758–8759;
- 18iB. M. Trost, M. K. Brennan, Org. Lett. 2006, 8, 2027–2030;
- 18jL. E. Overman, M. D. Rosen, Angew. Chem. 2000, 112, 4768–4771;
10.1002/1521-3757(20001215)112:24<4768::AID-ANGE4768>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4596–4599;10.1002/1521-3773(20001215)39:24<4596::AID-ANIE4596>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 18kP. R. Sebahar, R. M. Williams, J. Am. Chem. Soc. 2000, 122, 5666–5667.
- 19
- 19aY. K. Xu, S. P. Yang, S. G. Liao, H. Zhang, et al., J. Nat. Prod. 2006, 69, 1347–1350;
- 19bT. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, et al., J. Org. Chem. 2005, 70, 9430–9435;
- 19cR. F. Bond, J. C. A. Boeyens, C. W. Holzapfel, P. S. Steyn, J. Chem. Soc. Perkin Trans. 1 1979, 1751–1761;
- 19dsee Ref. [17b];
- 19eJ. Polonsky, M. A. Merrien, T. Prange, C. Pascard, S. Moreau, J. Chem. Soc. Chem. Commun. 1980, 601–602;
- 19fT. Prangé, M. A. Billion, M. Vuilhorgne, C. Pascard, J. Polonsky, S. Moreau, Tetrahedron Lett. 1981, 22, 1977–1980;
- 19gC. B. Cui, H. Kakeya, H. Osada, J. Antibiot. 1996, 49, 832–835;
- 19hH. Lin, S. J. Danishefsky, Angew. Chem. Int. Ed. 2003, 42, 36–51.
- 20C. Tratrat, S. Giorgi-Renault, H.-P. Husson, J. Org. Chem. 2000, 65, 6773–6776.
- 21S. G. Wierschke, J. Chandrasekhar, W. L. Jorgensen, J. Am. Chem. Soc. 1985, 107, 1496–1500.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.