Continuous-Flow Synthesis of Biaryls Enabled by Multistep Solid-Handling in a Lithiation/Borylation/Suzuki–Miyaura Cross-Coupling Sequence†
Dr. Wei Shu
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorDr. Laurent Pellegatti
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorDr. Matthias A. Oberli
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorDr. Wei Shu
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorDr. Laurent Pellegatti
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorDr. Matthias A. Oberli
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorW.S., L.P., and S.L.B. thank Novartis International AG for funding. M.A.O. acknowledges funding from the Novartis foundation. The Varian NMR instrument used was supported by the NSF (Grant Nos. CHE 9808061 and DBI 9729592).
Graphical Abstract
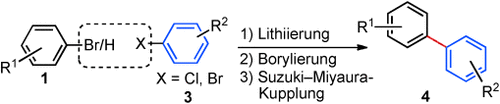
Immer stromabwärts: Mithilfe einer Sequenz aus Lithiierung, Borylierung und Suzuki-Miyaura-Kreuzkupplung unter kontinuierlichem Fluss lassen sich Biaryle bei Umgebungsbedingungen in effizienter und modularer Weise synthetisieren. Arylbromide und heterocyclische Vorstufen werden zunächst bei Raumtemperatur mit nBuLi lithiiert; dem folgen eine Borylierung sowie die Suzuki-Miyaura-Kupplung unter Ultraschall (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105223_sm_miscellaneous_information.pdf1.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews about flow chemistry, see:
- 1aJ. Wegner, S. Ceylan, A. Kirschning, Chem. Commun. 2011, 47, 4583;
- 1bC. Wiles, P. Watts, Chem. Commun. 2011, 47, 6512;
- 1cD. Webb, T. F. Jamison, Chem. Sci. 2010, 1, 675;
- 1dC. G. Frost, L. Mutton, Green Chem. 2010, 12, 1687;
- 1eA. Cukalovic, J. C. M. R. Monbaliu, C. V. Stevens, Top. Heterocycl. Chem. 2010, 23, 161;
- 1fR. L. Hartman, K. F. Jensen, Lab Chip 2009, 9, 2495;
- 1gK Geyer, T. Gustafsson, P. H. Seeberger, Synlett 2009, 2382;
- 1hJ.-i. Yoshida, A. Nagaki, T. Yamada, Chem. Eur. J. 2008, 14, 7450;
- 1iT. Fukuyama, M. T. Rahman, M. Sata, I. Ryu, Synlett 2008, 151;
- 1jB. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan, D. T. MaQuade, Chem. Rev. 2007, 107, 2300;
- 1kA. Kirschning, W. Solodenko, K. Mennecke, Chem. Eur. J. 2006, 12, 5972;
- 1lK. Geyer, J. D. C. Codée, P. H. Seeberger, Chem. Eur. J. 2006, 12, 8434;
- 1mK. Jähnisch, V. Hessel, H. Löwe, M. Baerns, Angew. Chem. 2004, 116, 410;
10.1002/ange.200300577 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 406.
- 2For books on microreactors, see:
- 2aY.-i. Yoshida, Flash Chemistry: Fast Organic Synthesis in Microsystems, Wiley-Blackwell, Hoboken, 2008;
10.1002/9780470723425 Google Scholar
- 2bT. Wirth, Microreactors in Organic Synthesis and Catalysis, Wiley-VCH, Weiheim, 2008;
10.1002/9783527622856 Google Scholar
- 2cP. H. Seeberger, T. Blume, New Avenues to Efficient Chemical Synthesis-Emerging Technologies, Springer, Berlin, 2007;
10.1007/978-3-540-70849-0 Google Scholar
- 2dW. Ehrfeld, V. Hessel, H. Löwe, Microreactors: New Technology for Modern Chemistry, Wiley-VCH, Weiheim, 2000.
10.1002/3527601953 Google Scholar
- 3
- 3aA. Sniady, M. W. Bedore, T. F. Jamison, Angew. Chem. 2011, 123, 2203; Angew. Chem. Int. Ed. 2011, 50, 2155;
- 3bT. Noël, S. Kuhn, A. J. Musacchio, K. F. Jensen, S. L. Buchwald, Angew. Chem. 2011, 123, 6065; Angew. Chem. Int. Ed. 2011, 50, 5943;
- 3cL. Malet-Sanz, J. Madrzak, S. V. Ley, I. R. Baxendale, Org. Biomol. Chem. 2010, 8, 5324;
- 3dM. D. Hopkin, I. R. Baxendale, S. V. Ley, Chem. Commun. 2010, 46, 2450;
- 3eA. Nagaki, A. Kenmoku, Y. Moriwaki, A. Hayashi, J.-i. Yoshida, Angew. Chem. 2010, 122, 7705;
10.1002/ange.201002763 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7543;
- 3fA. R. Bogdan, S. L. Poe, D. C. Kubis, S. J. Broadwater, D. T. McQude, Angew. Chem. 2009, 121, 8699;
10.1002/ange.200903055 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8547;
- 3gI. R. Baxendale, S. V. Ley, A. C. Mansfield, C. D. Smith, Angew. Chem. 2009, 121, 4077;
10.1002/ange.200900970 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4017;
- 3hT. P. Petersen, A. Ritzen, T. Ulven, Org. Lett. 2009, 11, 5134;
- 3iD. Grant, R. Dahl, N. D. P. Cosford, J. Org. Chem. 2008, 73, 7219;
- 3jM. Baumann, I. R. Baxendale, S. V. Ley, N. Nikbin, C. D. Smith, Org. Biomol. Chem. 2008, 6, 1587;
- 3kM. Baumann, I. R. Baxendale, S. V. Ley, N. Nikbin, C. D. Smith, J. Tierney, Org. Biomol. Chem. 2008, 6, 1577;
- 3lI. R. Baxendale, S. V. Ley, C. D. Smith, L. Tamborini, A. F. Voica, J. Comb. Chem. 2008, 10, 851;
- 3mT. Gustafsson, F. Ponten, P. H. Seeberger, Chem. Commun. 2008, 1100;
- 3nH. R. Sahoo, J. G. Kralj, K. F. Jensen, Angew. Chem. 2007, 119, 5806; Angew. Chem. Int. Ed. 2007, 46, 5704;
- 3oC. M. Griffiths-Jones, M. D. Hopkin, D. Jonsson, S. V. Ley, D. J. Tapolczay, E. Vickerstaffe, M. Ladlow, J. Comb. Chem. 2007, 9, 422;
- 3pI. R. Baxendale, J. Deeley, C. M. Griffiths-Jones, S. V. Ley, S. Saaby, G. K. Tranmer, Chem. Commun. 2006, 2566;
- 3qI. R. Baxendale, C. H. Griffiths-Jones, S. V. Ley, G. K. Tranmer, Synlett 2006, 42;
- 3rI. R. Baxendale, S. V. Ley, C. D. Smith, G. K. Tranmer, Chem. Commun. 2006, 4835;
- 3sI. R. Baxendale, C. M. Griffiths-Jones, S. V. Ley, G. K. Tranmer, Synlett 2006, 427;
- 3tS. France, D. Bernstein, A. Weatherwax, T. Lectka, Org. Lett. 2005, 7, 3009;
- 3uP. Watts, C. Wiles, S. J. Haswell, E. Pombo-Villar, Tetrahedron 2002, 58, 5427;
- 3vA. M. Hafez, A. E. Taggi, T. Dudding, T. Lectka, J. Am. Chem. Soc. 2001, 123, 10853.
- 4
- 4aT. Horie, M. Sumino, T. Tanaka, Y. Matsushita, T. Ichimura, J.-i. Yoshida, Org. Process Res. Dev. 2010, 14, 405;
- 4bJ. Sedelmeier, S. V. Ley, I. R. Baxendale, M. Baumann, Org. Lett. 2010, 12, 3618;
- 4cR. L. Hartman, J. R. Naber, N. Zaborenko, S. L. Buchwald, K. F. Jensen, Org. Process Res. Dev. 2010, 14, 1347;
- 4dT. Noël, J. R. Naber, R. L. Hartman, J. P. McMullen, K. F. Jensen, S. L. Buchwald, Chem. Sci. 2011, 2, 287;
- 4eS. Kuhn, T. Noël, L, Gu, P. L. Heider, K. F. Jensen, Lab Chip 2011, 11, 2488.
- 5
- 5a Metal-Catalyzed Cross-Coupling Reactions (Eds.: ), Wiley-VCH, New York, 1998;
- 5bS. P. Stanforth, Tetrahedron 1998, 54, 263;
- 5cJ. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359.
- 6
- 6aN. Miyaura, A. Suzuki, J. Chem. Soc. Chem. Commun. 1979, 866;
- 6bN. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Lett. 1979, 20, 3437;
- 6cN. Miyaura, T. Yanagi, A. Suzuki, Synth. Commun. 1981, 11, 513.
- 7For some selected reviews about the Suzuki–Miyaura cross-coupling, see:
- 7aC. A. Fleckensteinab, H. Plenio, Chem. Soc. Rev. 2010, 39, 694;
- 7bR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461;
- 7cG. A. Molander, N. M. Ellis, Acc. Chem. Res. 2007, 40, 275;
- 7dF. Bellina, A. Carpita, R. Rossi, Synthesis 2004, 2419;
- 7eN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457.
- 8For early examples on halogen–lithium exchange in flow, see:
- 8aX. Zhang, S. Stefanick, F. J. Villani, Org. Process Res. Dev. 2004, 8, 455;
- 8bT. Schwalbe, V. Autze, M. Hohmann, W. Stirner, Org. Process Res. Dev. 2004, 8, 440;
- 8cH. Usutani, Y. Tomida, A. Nagaki, H. Okamoto, T. Nokami, J.-i. Yoshida, J. Am. Chem. Soc. 2007, 129, 3046.
- 9
- 9aA. Pelter, K. Smith, H. C. Brown, Borane Reagents, Academic Press, London, 1988;
- 9bD. D. Winkle, K. M. Schaab, Org. Process Res. Dev. 2001, 5, 450;
- 9cM. M. Midland, Chem. Rev. 1989, 89, 1553;
- 9dK. Smith, A. Pelter in Comprehensive Organic Synthesis, Vol. 8 (Eds.: ), Pergamon, New York, 1991, p. 703;
- 9eD. S. Matteson, Reactivity and Structure Concept in Organic Synthesis: Stereodirected Synthesis with Organoboranes, Vol. 32, Springer, New York, 1994;
- 9fM. Vaultier, B. Carboni in Comprehensive Organometallic Chemistry, Vol. 11 (Eds.: ), Pergamon, New York, 1995, p. 191;
10.1016/B978-008046519-7.00096-4 Google Scholar
- 9gC. Ollivier, P. Renaud, Chem. Rev. 2001, 101, 3415;
- 9hM. Zaidlewicz, M. Krzeminski, Sci. Synth. 2004, 6, 1097;
- 9iV. Darmency, P. Renaud, Top. Curr. Chem. 2006, 263, 71.
- 10For direct transition-metal catalyzed borylations, see:
- 10aG. A. Molander, S. L. J. Trice, S. D. Dreher, J. Am. Chem. Soc. 2010, 132, 17701;
- 10bI. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890;
- 10cK. L. Billingsley, Stephen L. Buchwald, J. Org. Chem. 2008, 73, 5589;
- 10dK. Billingsley, T. E. Barder, S. L. Buchwald, Angew. Chem. 2007, 119, 5455;
10.1002/ange.200701551 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5359;
- 10eM. Murata, T. Sambommatsu, S. Watanabe, Y. Masuda, Synlett 2006, 1867;
- 10fT. Ishiyama, K. Ishida, N. Miyaura, Tetrahedron 2001, 57, 9813.
- 11During the preparation of this manuscript, an example of borates preparation at low temperature was reported: D. L. Browne, M. Baumann, B. H. Harji, I. R. Baxendale, S. V. Ley, Org. Lett. 2011, 13, 3312.
- 12For early examples on Pd-catalyzed coupling reactions in microflow, see:
- 12aG. M. Greenway, S. J. Haswell, D. O. Morgan, V. Skelton, P. Styring, Sens. Actuators B 2000, 63, 153;
- 12bT. Fukuyama, M. Shinmen, S. Nishitani, M. Sato, I. Ryu, Org. Lett. 2002, 4, 1691.
- 13B. A. Haag, C. Sämann, A. Jana, P. Knochel, Angew. Chem. 2011, 123, 7428;
10.1002/ange.201103022 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7290.
- 14T. Kinzel, Y. Zhang, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 14073, and references therein.
- 15For examples of the coupling of five-membered 2-heteroaromatic boronic acids, see:
- 15aY. Kabri, A. Gellis, P. Vanelle, Eur. J. Org. Chem. 2009, 4059;
- 15bG. S. Gill, D. W. Grobelny, J. H. Chaplin, B. L. Flynn, J. Org. Chem. 2008, 73, 1131;
- 15cM. Organ, S. Çalimsiz, M. Sayah, K. Hoi, A. Lough, Angew. Chem. 2009, 121, 2419; Angew. Chem. Int. Ed. 2009, 48, 2383;
- 15dY. Dang, Y. Chen, J. Org. Chem. 2007, 72, 6901;
- 15eH. Maeda, Y. Haketa, T. Nakanishi, J. Am. Chem. Soc. 2007, 129, 13661;
- 15fC. A. James, A. L. Coelho, M. Gevaert, P. Forgione, V. Snieckus, J. Org. Chem. 2009, 74, 4094.
- 16C. Giordiano, L. Coppi, F. Minisci, US Patent 5312975, 1994.
- 17J. Hannah, W. V. Ruyle, H. Jones, A. R. Matzuk, K. W. Kelly, B. E. Witzel, W. J. Holtz, R. A. Houser, T. Y. Shen, L. H. Sarett, J. Med. Chem. 1978, 21, 1093.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.