A Simple Enantioconvergent and Chemoenzymatic Synthesis of Optically Active α-Substituted Amides†
Dr. Wiktor Szymański
Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorAlja Westerbeek
Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dick B. Janssen
Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Dick B. Janssen, Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Ben L. Feringa, Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ben L. Feringa
Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Dick B. Janssen, Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Ben L. Feringa, Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorDr. Wiktor Szymański
Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorAlja Westerbeek
Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dick B. Janssen
Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Dick B. Janssen, Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Ben L. Feringa, Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ben L. Feringa
Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Dick B. Janssen, Department of Biochemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Ben L. Feringa, Center for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen (The Netherlands)
Search for more papers by this authorWe thank Theodora Tiemersma-Wegman and Monique Smith for their technical assistance with HPLC and GC analyses.
Graphical Abstract
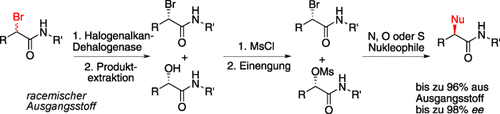
Einfach und effizient: Die Kombination einer enzymatischen, enantioinvertierenden Reaktion mit einfachen Folgeprozessen ermöglicht die Umwandlung racemischer Verbindungen in chirale α-substituierte Amide (siehe Bild; Ms=Methansulfonyl). Diese wichtigen Bausteine werden in hoher Gesamtausbeute und mit hohem Enantiomerenüberschuss erhalten; die entfallende Aufreinigung der Intermediate führt zu einem zeit- und kosteneffizienten Prozess.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105164_sm_miscellaneous_information.pdf295.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. P. Kokko, M. K. Hadden, K. S. Orwig, J. Mazella, T. A. Dix, J. Med. Chem. 2003, 46, 4141–4148.
- 2W. A. van der Linden, P. P. Geurink, C. Oskam, G. A. van der Marel, B. I. Florea, H. S. Overkleeft, Org. Biomol. Chem. 2010, 8, 1885–1893.
- 3V. P. Mocharla, B. Colasson, L. V. Lee, S. Röper, K. B. Sharpless, C.-H. Wong, H. C. Kolb, Angew. Chem. 2005, 117, 118;
10.1002/ange.200461580 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 116–120.
- 4W. Szymanski, R. Ostaszewski, Tetrahedron: Asymmetry 2006, 17, 2667–2671.
- 5W. Szymanski, M. Zwolinska, R. Ostaszewski, Tetrahedron 2007, 63, 7647–7653.
- 6W. Szymanski, R. Ostaszewski, Tetrahedron 2008, 64, 3197–3203.
- 7J. F. Fisher, S. Mobashery, Cancer Metastasis Rev. 2006, 25, 115–136.
- 8S. Hanessian, L. Auzzas, A. Larsson, J. B. Zhang, G. Giannini, G. Gallo, A. Ciacci, W. Cabri, ACS Med. Chem. Lett. 2010, 1, 70–74.
- 9S. V. Andurkar, J. P. Stables, H. Kohn, Bioorg. Med. Chem. 1999, 7, 2381–2389.
- 10A. J. Lopez-Farre, D. Sacristan, J. J. Zamorano-Leon, N. San-Martin, C. Macaya, Cardiovasc. Ther. 2008, 26, 65–74.
- 11B. Barlaam, T. G. Bird, C. Lambert-van der Brempt, D. Campbell, S. J. Foster, R. Maciewicz, J. Med. Chem. 1999, 42, 4890–4908.
- 12J. T. Repine, J. S. Kaltenbronn, A. M. Doherty, J. M. Hamby, R. J. Himmelsbach, B. E. Kornberg, M. D. Taylor, E. A. Lunney, C. Humblet, S. T. Rapundalo, B. L. Batley, M. J. Ryan, C. A. Painchaud, J. Med. Chem. 1992, 35, 1032–1042.
- 13Y. Simeo, W. Kroutil, K. Faber in Enzymes in Action: Green Solutions for Chemical Problems (Eds.: ), Kluwer Academic Publishers, Dordrecht, 2000, pp. 27–51.
- 14L. Cao, J. T. Lee, W. Chen, T. K. Wood, Biotechnol. Bioeng. 2006, 94, 522–529.
- 15K. M. Manoj, A. Archelas, J. Baratti, R. Furstoss, Tetrahedron 2001, 57, 695–701.
- 16E. Y. Lee, M. L. Shuler, Biotechnol. Bioeng. 2007, 98, 318–327.
- 17S. Hwang, C. Y. Choi, E. Y. Lee, Biotechnol. Lett. 2008, 30, 1219–1225.
- 18M. I. Monterde, M. Lombard, A. Archelas, A. Cronin, M. Arand, R. Furstoss, Tetrahedron: Asymmetry 2004, 15, 2801–2805.
- 19H. Danda, T. Nagatomi, A. Maehara, T. Umemura, Tetrahedron 1991, 47, 8701–8716.
- 20K. Lemke, S. Ballschuh, A. Kunath, F. Theil, Tetrahedron: Asymmetry 1997, 8, 2051–2055.
- 21S. Takano, M. Suzuki, K. Ogasawara, Tetrahedron: Asymmetry 1993, 4, 1043–1046.
- 22S. R. Wallner, M. Pogorevc, H. Trauthwein, K. Faber, Eng. Life Sci. 2004, 4, 512–516.
- 23P. Gadler, T. C. Reiter, K. Hoelsch, D. Weuster-Botz, K. Faber, Tetrahedron: Asymmetry 2009, 20, 115–118.
- 24M. Schober, P. Gadler, T. Knaus, H. Kayer, R. Birner-Grünberger, C. Gülly, P. Macheroux, U. Wagner, K. Faber, Org. Lett. 2011, 13, 4296–4299.
- 25D. B. Janssen, Curr. Opin. Chem. Biol. 2004, 8, 150–159.
- 26A. Westerbeek, W. Szymański, H. J. Wijma, S. J. Marrink, B. L. Feringa, D. B. Janssen, Adv. Synth. Catal. 2011, 353, 931–944.
- 27E. Vänttinen, L. Kanerva, Tetrahedron: Asymmetry 1995, 6, 1779–1786.
- 28S. Pedragosa-Moreau, C. Morisseau, J. Baratti, J. Zylber, A. Archelas, R. Furstoss, Tetrahedron 1997, 53, 9707–9714.
- 29D. J. Cram, Fundamentals of Carbanion Chemistry, Academic Press, New York, 1965, pp. 71–84.
- 30C. F. Bernasconi, K. W. Kittredge, J. Org. Chem. 1998, 63, 1944–1953.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.