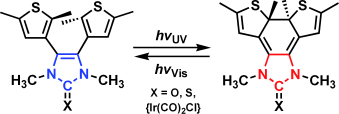
Photoswitchable N-Heterocyclic Carbenes: Using Light to Modulate Electron-Donating Properties†
Bethany M. Neilson
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Search for more papers by this authorVincent M. Lynch
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Search for more papers by this authorCorresponding Author
Prof. Christopher W. Bielawski
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.eduSearch for more papers by this authorBethany M. Neilson
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Search for more papers by this authorVincent M. Lynch
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Search for more papers by this authorCorresponding Author
Prof. Christopher W. Bielawski
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.edu
Department of Chemistry & Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX 78712 (USA) http://bielawski.cm.utexas.eduSearch for more papers by this authorWe are grateful to the ARO (W911NF-09-1-0446), the NSF (CHE-0645563), and the Welch Foundation (F-1621) for their generous support.
Graphical Abstract
Mit Licht wurden die Elektronendonor-Eigenschaften eines N-heterocylischen Carbens (NHC) als Teil von Chalkogen- und Metalladdukten verändert. Ein lichtinduzierter elektrocyclischer Ringschluss eines photochromen 4,5-Dithienylimidazolons erhöhte dessen νCO-Frequenz. UV-Bestrahlung des analogen photochromen Komplexes [(NHC)Ir(CO)2Cl] verringerte die Elektronendonor-Fähigkeit des Carbens. Mit sichtbarem Licht ließen sich beide Photocyclisierungen wieder umkehren.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105032_sm_miscellaneous_information.pdf5.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on NHCs and their coordination chemistry, see:
- 1aF. E. Hahn, M. C. Jahnke, Angew. Chem. 2008, 120, 3166–3216; Angew. Chem. Int. Ed. 2008, 47, 3122–3172;
- 1bW. A. Herrmann, Angew. Chem. 2002, 114, 1342–1363;
Angew. Chem. Int. Ed. 2002, 41, 1290–1309;
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 1cD. Bourissou, O. Guerret, F. P. Gabbaï, G. Bertrand, Chem. Rev. 2000, 100, 39–91;
- 1dW. A. Herrmann, C. Köcher, Angew. Chem. 1997, 109, 2256–2282; Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187;
- 1eS. Díez-González, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612–3676;
- 1fT. Dröge, F. Glorius, Angew. Chem. 2010, 122, 7094–7107;
10.1002/ange.201001865 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6940–6952.
- 2For studies of tuning the electron-donating ability of NHCs as a means to influence catalytic reactions, see:
- 2aD. M. Khramov, E. L. Rosen, J. A. V. Er, P. D. Vu, V. M. Lynch, C. W. Bielawski, Tetrahedron 2008, 64, 6853–6862;
- 2bM. D. Sanderson, J. W. Kamplain, C. W. Bielawski, J. Am. Chem. Soc. 2006, 128, 16514–16515;
- 2cD. M. Khramov, V. M. Lynch, C. W. Bielawski, Organometallics 2007, 26, 6042;
- 2dL. Benhamou, N. Vujkovic, V. César, H. Gornitzka, N. Lugan, G. Lavigne, Organometallics 2010, 29, 2616–2630;
- 2eM. Süßner, H. Plenio, Chem. Commun. 2005, 5417–5419;
- 2fV. Sashuk, L. H. Peeck, H. Plenio, Chem. Eur. J. 2010, 16, 3983–3993;
- 2gT. W. Hudnall, J. P. Moerdyk, C. W. Bielawski, Chem. Commun. 2010, 46, 4288–4290;
- 2hJ. P. Moerdyk, C. W. Bielawski, Organometallics 2011, 30, 2278–2284;
- 2iA. Fürstner, M. Alcarazo, H. Krause, C. W. Lehmann, J. Am. Chem. Soc. 2007, 129, 12676–12677;
- 2jT. W. Hudnall, A. G. Tennyson, C. W. Bielawksi, Organometallics 2010, 29, 4569–4578;
- 2kC. J. O’Brien, E. A. B. Kantchev, G. A. Chass, H. Niloufar, A. C. Hopkinson, M. G. Organ, D. H. Setiadi, T. Tang, D. Fan, Tetrahedron 2005, 61, 9723–9735.
- 3For examples of switchable NHCs and related catalysts, see:
- 3aA. G. Tennyson, V. M. Lynch, C. W. Bielawski, J. Am. Chem. Soc. 2010, 132, 9420–9429;
- 3bM. Süßner, H. Plenio, Angew. Chem. 2005, 117, 7045–7048;
10.1002/ange.200502182 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6885–6888;
- 3cE. L. Rosen, C. D. Varnado, Jr., A. G. Tennyson, D. M. Khramov, J. W. Kamplain, D. H. Sung, P. T. Cresswell, V. M. Lynch, C. W. Bielawski, Organometallics 2009, 28, 6695–6706;
- 3dL. H. Peeck, S. Leuthäusser, H. Plenio, Organometallics 2010, 29, 4339–4345;
- 3eS. L. Balof, S. J. P’Pool, N. J. Berger, E. J. Valente, A. M. Shiller, H.-J. Schanz, Dalton Trans. 2008, 5791–5799;
- 3fS. L. Balof, B. Yu, A. B. Lowe, Y. Ling, Y. Zhang, H.-J. Schanz, Eur. J. Inorg. Chem. 2009, 1717–1722;
- 3gS. J. P’Pool, H.-J. Schanz, J. Am. Chem. Soc. 2007, 129, 14200–14212.
- 4
- 4a Photochromism: Molecules and Systems (Eds.: ), Elsevier, Amsterdam, 2003;
- 4bSpecial Issue: “Photochromism: Memories and Switches” (Ed.: M. Irie), Chem. Rev. 2000, 100, 1683.
- 5
- 5aM. Irie, Chem. Rev. 2000, 100, 1685–1716;
- 5bK. Matsuda, M. Irie, J. Photochem. Photobiol. C 2004, 169–182;
- 5cH. Tian, S. Yang, Chem. Soc. Rev. 2004, 33, 85–97;
- 5dH. Tian, S. Wang, Chem. Commun. 2007, 781–792;
- 5eC. Yun, J. You, J. Kim, J. Huh, E. Kim, J. Photochem. Photobiol. C 2009, 10, 111–129;
- 5fM. Akita, Organometallics 2011, 30, 43–51.
- 6For an investigation of the photophysical properties of metal complexes supported by 1,3-dimethyl-4,5-dithienylimidazolylidenes, see:
- 6aV. W.-W. Yam, J. K. Lee, C. Ko, N. Zhu, J. Am. Chem. Soc. 2009, 131, 912–913;
- 6bP. H.-M. Lee, C.-C. Ko, N. Zhu, V. W.-W. Yam, J. Am. Chem. Soc. 2007, 129, 6058–6059.
- 7
- 7aA. R. Siamaki, D. A. Black, B. A. Arndtsen, J. Org. Chem. 2008, 73, 1135–1138.
- 8In support of this hypothesis, ring-opened 4,5-dithienylimidazolium salts were found to be more electrophilic than their ring-closed isomers; see:
- 8aG. Duan, N. Zhu, V. W.-W. Yam, Chem. Eur. J. 2010, 16, 13199–13209;
- 8bT. Nakashima, M. Goto, S. Kawai, T. Kawai, J. Am. Chem. Soc. 2008, 130, 14570–14575.
- 9
- 9aY. Odo, K. Matsuda, M. Irie, Chem. Eur. J. 2006, 12, 4283–4288;
- 9bV. Lemieux, M. D. Spantulescu, K. K. Baldridge, N. R. Branda, Angew. Chem. 2008, 120, 5112–5115;
10.1002/ange.200800869 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5034–5037;
- 9cH. D. Samachetty, N. R. Branda, Chem. Commun. 2005, 2840;
- 9dD. Sud, T. B. Norsten, N. R. Branda, Angew. Chem. 2005, 117, 2055–2057;
10.1002/ange.200462538 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2019–2021;
- 9eM. Herder, M. Pätzel, L. Grubert, Hecht, Chem. Commun. 2011, 47, 460–462;
- 9fS. H. Kawai, S. L. Gilat, J. M. Lehn, Eur. J. Org. Chem. 1999, 2359–2366;
10.1002/(SICI)1099-0690(199909)1999:9<2359::AID-EJOC2359>3.0.CO;2-# CAS Web of Science® Google Scholar
- 9gV. Lemieux, S. Gauthier, N. R. Branda, Angew. Chem. 2006, 118, 6974–6978;
10.1002/ange.200601584 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6820–6824;
- 9hD. Sud, T. J. Wigglesworth, N. R. Branda, Angew. Chem. 2007, 119, 8163–8165;
10.1002/ange.200703034 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8017–8019;
- 9iH. D. Samachetty, V. Lemieux, N. R. Branda, Tetrahedron 2008, 64, 8292–8300;
- 9jR. S. Stoll, S. Hecht, Angew. Chem. 2010, 122, 5176–5200;
10.1002/ange.201000146 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5054–5075;
- 9kT. Hirose, K. Matsuda, M. Irie, J. Org. Chem. 2006, 71, 7499–7508;
- 9lT. Hirose, M. Irie, K. Matsuda, Adv. Mater. 2008, 20, 2137–2141;
- 9mM. Singer, A. Jäschke, J. Am. Chem. Soc. 2010, 132, 8372–8377.
- 10
- 10aD. Sud, R. McDonald, N. R. Branda, Inorg. Chem. 2005, 44, 5960–5962;
- 10bY. Tanaka, A. Inagaki, M. Akita, Chem. Commun. 2007, 1169–1171;
- 10cK. Motoyama, T. Koike, M. Akita, Chem. Commun. 2008, 5812–5814;
- 10dY. Tanaka, A. Inagaki, T. Koike, C. Lapinte, M. Akita, Chem. Eur. J. 2010, 16, 4762–4776;
- 10eK. Uchida, A. Inagaki, M. Akita, Organometallics 2007, 26, 5030–5041.
- 11For other examples of photochromic 4,5-diarylimidazolones, see: M. M. Krayushkin, S. N. Ivanov, A. Y. Martynkin, B. V. Lichitsky, A. A. Dudinov, L. G. Vorontsova, Z. A. Starikova, B. M. Uzhinov, Russ. Chem. Bull. Int. Ed. 2002, 51, 1731–1736.
- 12L. I. Belen’kii, V. Z. Shirinyan, G. P. Gromova, A. V. Kolotaev, Y. A. Strelenko, S. N. Tandura, A. N. Shumskii, M. M. Krayushkin, Chem. Heterocycl. Compd. 2003, 39, 1570–1578.
- 13F. Toda, K. Tanaka, H. Tange, J. Chem. Soc. Perkin Trans. 1 1989, 1555–1556.
- 14B. L. Benac, E. M. Burgess, A. J. Arduengo, Org. Synth. 1986, 64, 92.
- 15The conversion of the photocyclization reaction involving 1 was determined using the molar absorptivities of 1 o and 1 c. The conversion of the reaction involving 2 was determined using the molar absorptivity of 2 c, as obtained from a combination of quantitative 1H NMR and UV/Vis spectroscopy.
- 16Based on the extinction coefficient for the absorbance measured at 240 nm, >90 % of the mixture returns to 1 o; see Supporting Information.
- 17Urea 1 o exhibited a νCO typical of amides while that of 1 c was more characteristic of aliphatic ketones; see: E. Pretsch, P. Buhlmann, M. Badertscher, Structure Determination of Organic Compounds: Tables of Spectral Data, 4th ed., Springer, Berlin, 2009, pp. 310–324.
- 18
- 18aC. N. R. Rao, Chemical Applications of Infrared Spectroscopy, Academic Press, New York, 1963, pp. 298–303;
- 18bM. Davies, J. Jones, J. Chem. Soc. 1958, 955–958.
- 19See Supporting Information for UV/Vis spectral data for the photocycloreversion of isolated 1 c to 1 o.
- 20See Supporting Information for additional details of the X-ray diffraction analyses. CCDC 821251 (1 o) and CCDC 821252 (1 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21The sum of the following angles was calculated to be 324°: C4-C7-C14, C14-C7-S1, C4-C7-S1.
- 22R. B. Woodward, R. Hoffmann, The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970.
- 23The solid-state structure of 1 c was slightly disordered due to the existence of two enantiomers in the sample, with one having the S,S configuration at C6 and C7 and the other having the R,R configuration at the same atoms. See Supporting Information for additional discussion. Similar disorder has been observed in previously reported structures of ring-closed perfluorocyclopentene-based dithienylethenes; see:
- 23aS. Kobatake, M. Yamada, T. Yamada, M. Irie, J. Am. Chem. Soc. 1999, 121, 8450–8456;
- 23bM. Morimoto, M. Irie, J. Am. Chem. Soc. 2010, 132, 14172–14178;
- 23cH. Choi, H. Lee, Y. Kang, E. Kim, S.-O. Kang, J. Ko, J. Org. Chem. 2005, 70, 8291–8297;
- 23dM. Irie, T. Lifka, S. Kobatake, N. Kato, J. Am. Chem. Soc. 2000, 122, 4871–4876;
- 23eS. Kobatake, K. Shibata, K. Uchida, M. Irie, J. Am. Chem. Soc. 2000, 122, 12135–12141;
- 23fM. Irie, S. Kobatake, M. Horichi, Science 2001, 291, 1769–1772;
- 23gM. Morimoto, S. Kobatake, M. Irie, Chem. Eur. J. 2003, 9, 621–627;
- 23hM. Morimoto, S. Kobatake, M. Irie, Photochem. Photobiol. Sci. 2003, 2, 1088–1094;
- 23iT. Kodani, K. Matsuda, T. Yamada, S. Kobatake, M. Irie, J. Am. Chem. Soc. 2000, 122, 9631–9637.
- 24Nearly identical percent buried volumes (%Vbur) were calculated for 1 o (30.1 %) and 1 c (30.2 %). The calculations were performed using the web application SamVca with Bondi radii scaled by 1.17, R=3.5 Å for the sphere radius, d=2.10 Å for the distance between C1 and O1, and s=0.05 Å for the mesh spacing; see: A. Poater, B. Cosenza, A. Correa, S. Giudice, F. Ragone, V. Scarano, L. Cavallo, Eur. J. Inorg. Chem. 2009, 1759–1766.
- 25
- 25aA. R. Chianese, X. Li, M. C. Janzen, J. W. Faller, R. H. Crabtree, Organometallics 2003, 22, 1663–1667;
- 25bH. M. J. Wang, I. J. B. Lin, Organometallics 1998, 17, 972–975.
- 26
- 26aR. A. Kelly III, H. Clavier, S. Giudice, N. M. Scott, E. D. Stevens, J. Bordner, I. Samardjiev, C. D. Hoff, L. Cavallo, S. P. Nolan, Organometallics 2008, 27, 202–210;
- 26bC. A. Tolman, Chem. Rev. 1977, 77, 313–348.
- 27Decarbonylation was observed by IR spectroscopy when 3 o was irradiated in acetonitrile or dichloromethane; see Supporting Information.
- 28See Supporting Information for the UV/Vis spectral data accompanying the cyclization of 3 o to 3 c.
- 29The conversion of 3 o to 3 c was calculated to be 18 % by IR spectroscopy after 10 min of irradiation at 297 nm. After 45 min of irradiation, the conversion was calculated to be 29 % by IR spectroscopy (see Supporting Information). These conversions are in accord with other reported photochromic DAE-containing metal complexes; see:
- 29aS. Kume, H. Nishihara, Dalton Trans. 2008, 3260–3271;
- 29bW. Tan, J. Zhou, F. Li, T. Yi, H. Tian, Chem. Asian J. 2011, 6, 1263–1268;
- 29cRef. [6a].
- 30The results reported herein demonstrate that the bonding characteristics of carbamides may be modulated using only electronics as opposed to stereoelectronic approaches; see:
- 30aC. Chan, R. Heid, S. Zheng, J. Guo, B. Zhou, T. Furuuchi, S. J. Danishefsky, J. Am. Chem. Soc. 2005, 127, 4596–4598;
- 30bJ. Clayden, W. Moran, Angew. Chem. 2006, 118, 7276–7278; Angew. Chem. Int. Ed. 2006, 45, 7118–7120;
- 30cK. Tani, B. M. Stoltz, Nature 2006, 441, 731–734;
- 30dM. Szostak, L. Yao, V. W. Day, D. R. Powell, J. Aubé, J. Am. Chem. Soc. 2010, 132, 8836–8837;
- 30eT. W. Hudnall, C. W. Bielawski, J. Am. Chem. Soc. 2009, 131, 16039–16041.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.