Anion-Assisted Supramolecular Polymerization: From Achiral AB-Type Monomers to Chiral Assemblies†
Dr. Calogero Capici
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Search for more papers by this authorProf. Yoram Cohen
School of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv 69978, Tel Aviv (Israel)
Search for more papers by this authorDr. Alessandro D'Urso
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Dr. Giuseppe Gattuso
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorDr. Anna Notti
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Search for more papers by this authorDr. Andrea Pappalardo
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Sebastiano Pappalardo
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Melchiorre F. Parisi
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Roberto Purrello
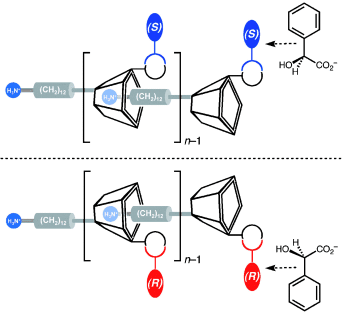
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorDr. Sarit Slovak
School of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv 69978, Tel Aviv (Israel)
Search for more papers by this authorDr. Valentina Villari
CNR-IPCF, Istituto per i Processi Chimico-Fisici, Viale F. Stagno d'Alcontres 37, 98158 Messina (Italy)
Search for more papers by this authorDr. Calogero Capici
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Search for more papers by this authorProf. Yoram Cohen
School of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv 69978, Tel Aviv (Israel)
Search for more papers by this authorDr. Alessandro D'Urso
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Dr. Giuseppe Gattuso
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorDr. Anna Notti
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Search for more papers by this authorDr. Andrea Pappalardo
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Sebastiano Pappalardo
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Melchiorre F. Parisi
Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorCorresponding Author
Prof. Roberto Purrello
Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Giuseppe Gattuso, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Sebastiano Pappalardo, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Melchiorre F. Parisi, Dipartimento di Chimica Organica e Biologica, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina (Italy)
Roberto Purrello, Dipartimento di Scienze Chimiche, Università di Catania, Viale A. Doria 6, 95125 Catania (Italy)
Search for more papers by this authorDr. Sarit Slovak
School of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv 69978, Tel Aviv (Israel)
Search for more papers by this authorDr. Valentina Villari
CNR-IPCF, Istituto per i Processi Chimico-Fisici, Viale F. Stagno d'Alcontres 37, 98158 Messina (Italy)
Search for more papers by this authorMIUR (PRIN-2009A5Y3N9 project) is gratefully acknowledged for financial support of this research.
Graphical Abstract
Anionen machen den Unterschied! Gegenionen, die während der Säure-geförderten Selbstorganisation von Monomervorstufen des AB-Typs freigesetzt werden, docken an designierte Hilfsbindungsstellen an und erleichtern somit den Poymerisierungsprozess, während sie ihre molekularen Eigenschaften auf die gesamte supramolekulare Struktur übertragen (siehe Bild).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104357_sm_miscellaneous_information.pdf4.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Brunsveld, B. J. B. Folmer, E. W. Meijer, R. P. Sijbesma, Chem. Rev. 2001, 101, 4071–4098;
- 1bJ.-M. Lehn, Polym. Int. 2002, 51, 825–839;
- 1c Supramolecular Polymers, 2nd ed. ), CRC, Boca Raton, FL, 2005.
- 2M. A. Cohen Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov, S. Minko, Nat. Mater. 2010, 9, 101–113.
- 3
- 3aP. Cordier, F. Tournilhac, C. Soulie-Ziakovic, L. Lieber, Nature 2008, 451, 977–980;
- 3bM. D. Hager, P. Greil, C. Leyens, S. van der Zwaag, U. S. Schubert, Adv. Mater. 2010, 22, 5424–5430.
- 4
- 4aT. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma, E. W. Meijer, Chem. Rev. 2009, 109, 5687–5754;
- 4bD. González-Rodríguez, A. P. H. J. Schenning, Chem. Mater. 2011, 23, 310–325.
- 5
- 5aT. Haino, Y. Matsumoto, Y. Fukazawa, J. Am. Chem. Soc. 2005, 127, 8936–8937;
- 5bF. Huang, J. W. Jones, Prog. Polym. Sci. 2005, 30, 982–1018;
- 5cG. Gattuso, A. Notti, A. Pappalardo, M. F. Parisi, I. Pisagatti, S. Pappalardo, D. Garozzo, A. Messina, Y. Cohen, S. Slovak, J. Org. Chem. 2008, 73, 7280–7289;
- 5dA. Harada, Y. Takashima, H. Yamaguchi, Chem. Soc. Rev. 2009, 38, 875–882;
- 5eA. Harada, A. Hashidzume, H. Yamaguchi, Y. Takashima, Chem. Rev. 2009, 109, 5974–6023;
- 5fT. Haino, E. Hirai, Y. Fujiwara, K. Kashihara, Angew. Chem. 2010, 122, 8071–8075;
10.1002/ange.201004074 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7899–7903;
- 5gZ. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma, F. Huang, Angew. Chem. 2011, 123, 1433–1437; Angew. Chem. Int. Ed. 2011, 50, 1397–1401.
- 6S. Pappalardo, V. Villari, S. Slovak, Y. Cohen, G. Gattuso, A. Notti, A. Pappalardo, I. Pisagatti, M. F. Parisi, Chem. Eur. J. 2007, 13, 8164–8173.
- 7
- 7aF. Arnaud-Neu, S. Fuangswasdi, A. Notti, S. Pappalardo, M. F. Parisi, Angew. Chem. 1998, 110, 120–122;
10.1002/(SICI)1521-3757(19980116)110:1/2<120::AID-ANGE120>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1998, 37, 112–114;10.1002/(SICI)1521-3773(19980202)37:1/2<112::AID-ANIE112>3.0.CO;2-O CAS Web of Science® Google Scholar
- 7bD. Garozzo, G. Gattuso, F. H. Kohnke, P. Malvagna, A. Notti, S. Occhipinti, S. Pappalardo, M. F. Parisi, I. Pisagatti, Tetrahedron Lett. 2002, 43, 7663–7667.
- 8G. Gattuso, A. Notti, S. Pappalardo, M. F. Parisi, T. Pilati, G. Resnati, G. Terraneo, T. Pilati, CrystEngComm 2009, 11, 1204–1206.
- 9At a 40 mM monomer concentration the number-average degree of polymerization (
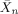 ) was found to be approximately 40 and 2 in the case of the picrate and chloride derivatives, respectively; see Ref. [6].
) was found to be approximately 40 and 2 in the case of the picrate and chloride derivatives, respectively; see Ref. [6].
- 10
- 10aJ. W. Jones, H. W. Gibson, J. Am. Chem. Soc. 2003, 125, 7001–7004;
- 10bF. Huang, J. W. Jones, C. Slebodnik, H. W. Gibson, J. Am. Chem. Soc. 2003, 125, 14458–14464;
- 10cT. B. Gasa, J. M. Spruell, W. R. Dichtel, T. J. Sørensen, D. Philp, J. F. Stoddart, P. Kuzmic, Chem. Eur. J. 2009, 15, 106–116;
- 10dS. Roelens, A. Vacca, O. Francesconi, C. Venturi, Chem. Eur. J. 2009, 15, 8296–8302;
- 10eS. K. Kim, J. L. Sessler, Chem. Soc. Rev. 2010, 39, 3784–3809;
- 10fT. B. Gasa, C. Valente, J. F. Stoddart, Chem. Soc. Rev. 2011, 40, 57–78.
- 11For representative examples, see:
- 11aP. R. Ashton, I. W. Parsons, F. M. Raymo, J. F. Stoddart, A. J. P. White, D. J. Williams, R. Wolf, Angew. Chem. 1998, 110, 2016–2019;
10.1002/(SICI)1521-3757(19980703)110:13/14<2016::AID-ANGE2016>3.0.CO;2-4 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1913–1916;10.1002/(SICI)1521-3773(19980803)37:13/14<1913::AID-ANIE1913>3.0.CO;2-W CAS Web of Science® Google Scholar
- 11bN. Yamaguchi, D. S. Nagvekar, H. W. Gibson, Angew. Chem. 1998, 110, 2518–2520;
10.1002/(SICI)1521-3757(19980904)110:17<2518::AID-ANGE2518>3.0.CO;2-1 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2361–2364;10.1002/(SICI)1521-3773(19980918)37:17<2361::AID-ANIE2361>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 11cN. Yamaguchi, H. W. Gibson, Angew. Chem. 1999, 111, 195–199;
Angew. Chem. Int. Ed. 1999, 38, 143–147;
10.1002/(SICI)1521-3773(19990115)38:1/2<143::AID-ANIE143>3.0.CO;2-Y CAS Web of Science® Google Scholar
- 11dF. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li, F. Huang, J. Am. Chem. Soc. 2008, 130, 11254–11255;
- 11eX. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shaod, F. Huang, Chem. Commun. 2011, 47, 7086–7088.
- 12For representative examples, see:
- 12aF. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, L. Wu, Y. Yu, H. W. Gibson, F. Huang, Angew. Chem. 2010, 122, 1108–1112; Angew. Chem. Int. Ed. 2010, 49, 1090–1094;
- 12bY.-S. Su, J.-W. Liu, Y. Jiang, C.-F. Chen, Chem. Eur. J. 2011, 17, 2435–2441;
- 12cZ. Niu, F. Huang, H. W. Gibson, J. Am. Chem. Soc. 2011, 133, 2836–2839.
- 13For representative examples, see:
- 13aK. Ohga, Y. Takashima, H. Takahashi, Y. Kawaguchi, H. Yamaguchi, A. Harada, Macromolecules 2005, 38, 5897–5904;
- 13bV. H. Soto Tellini, A. Jover, J. Carrazana García, L. Galantini, F. Meijide, J. Vázquez Tato, J. Am. Chem. Soc. 2006, 128, 5728–5734;
- 13cP. Kuad, A. Miyawaki, Y. Takashima, H. Yamaguchi, A. Harada, J. Am. Chem. Soc. 2007, 129, 12630–12631.
- 14For representative examples, see:
- 14aU. Rauwald, O. A. Scherman, Angew. Chem. 2008, 120, 4014–4017;
10.1002/ange.200705591 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3950–3953;
- 14bY. Liu, Y. Yu, J. Gao, Z. Wang, X. Zhang, Angew. Chem. 2010, 122, 6726–6729; Angew. Chem. Int. Ed. 2010, 49, 6576–6579.
- 15D.-S. Guo, S. Chen, H. Qian, H.-Q. Zhang, Y. Liu, Chem. Commun. 2010, 46, 2620–2622.
- 16For examples of calixarene-based supramolecular polymers relying on multiple-hydrogen bonds as well as guest encapsulation, see:
- 16aR. K. Castellano, C. Nuckolls, S. H. Eichhorn, M. R. Wood, A. J. Lovinger, J. Rebek, Jr., Angew. Chem. 1999, 111, 2764–2768;
10.1002/(SICI)1521-3757(19990903)111:17<2764::AID-ANGE2764>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2603–2606;10.1002/(SICI)1521-3773(19990903)38:17<2603::AID-ANIE2603>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 16bH. Xu, D. M. Rudkevich, Chem. Eur. J. 2004, 10, 5432–5442.
- 17
- 17aR. M. Yebeutchou, F. Tancini, N. Demitri, S. Geremia, R. Mendichi, E. Dalcanale, Angew. Chem. 2008, 120, 4580–4584;
10.1002/ange.200801002 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4504–4508;
- 17bF. Tancini, R. M. Yebeutchou, L. Pirondini, R. De Zorzi, S. Geremia, O. A. Scherman, E. Dalcanale, Chem. Eur. J. 2010, 16, 14313–14321.
- 18G. Cafeo, G. Gattuso, F. H. Kohnke, A. Notti, S. Occhipinti, S. Pappalardo, M. F. Parisi, Angew. Chem. 2002, 114, 2226–2230;
10.1002/1521-3757(20020617)114:12<2226::AID-ANGE2226>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2122–2126.10.1002/1521-3773(20020617)41:12<2122::AID-ANIE2122>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 19
- 19aG. Gattuso, C. Gargiulli, C. Liotta, A. Notti, M. F. Parisi, I. Pisagatti, S. Pappalardo, J. Org. Chem. 2009, 74, 4350–4353;
- 19bC. Capici, R. De Zorzi, C. Gargiulli, G. Gattuso, S. Geremia, A. Notti, S. Pappalardo, M. F. Parisi, F. Puntoriero, Tetrahedron 2010, 66, 4987–4993.
- 20D. Garozzo, G. Gattuso, A. Notti, A. Pappalardo, S. Pappalardo, M. F. Parisi, M. Perez, I. Pisagatti, Angew. Chem. 2005, 117, 4970–4974;
10.1002/ange.200500985 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4892–4896.
- 21
- 21aB. P. Hay, T. K. Firman, B. A. Moyer, J. Am. Chem. Soc. 2005, 127, 1810–1819;
- 21bV. Amendola, L. Fabbrizzi, L. Mosca, Chem. Soc. Rev. 2010, 39, 3889–3915.
- 22See the Supporting Information for experimental details.
- 23
- 23aD. R. Stewart, M. Krawiec, R. P. Kashyap, W. H. Watson, C. D. Gutsche, J. Am. Chem. Soc. 1995, 117, 586–601;
- 23bG. De Salvo, G. Gattuso, A. Notti, M. F. Parisi, S. Pappalardo, J. Org. Chem. 2002, 67, 684–692.
- 24For previous examples of endo-cavity inclusion of alkylammonium and alkanediyldiammonium ions inside calix[5]arene derivatives, see: Refs. [6–8] and [18–20].
- 25The alternative process of endo-cavity intramolecular inclusion of the pendant chain had been previously ruled out by 1H NMR diffusion experiments on the protonated form of C5-NH2 (see Ref. [6]). For an example of self-threading, see: Y. Inoue, M. Miyauchi, H. Nakajima, Y. Takashima, H. Yamaguchi, A. Harada, J. Am. Chem. Soc. 2006, 128, 8994–8995.
- 26Signal assignments, reported in Figure 1, follow from DQF-COSY and 1D TOCSY experiments (see the Supporting Information).
- 27For a review on the application of diffusion NMR spectroscopy in supramolecular chemistry, see: Y. Cohen, L. Avram, L. Frish, Angew. Chem. 2005, 117, 524–560;
10.1002/ange.200300637 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 520–554. For a selection of recent articles on the same topic see:
- 27aL. Avram, Y. Cohen, J. Am. Chem. Soc. 2004, 126, 11556–11563;
- 27bT. Evan-Salem, I. Baruch, L. Avram, L. C. Palmer, J. Rebek, Jr., Proc. Natl. Acad. Sci. USA 2006, 103, 12296–12300;
- 27cT. Evan-Salem, L. Frish, F. W. B. van Leeuwen, D. N. Reinhoudt, W. Verboom, M. S. Kaucher, J. T. Davis, Y. Cohen, Chem. Eur. J. 2007, 13, 1969–1977;
- 27dL. Avram, Y. Cohen, Org. Lett. 2008, 10, 1505–1508;
- 27eS. Slovak, L. Avram, Y. Cohen, Angew. Chem. 2010, 122, 438–441;
10.1002/ange.200904952 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 428–431;
- 27fA. Lledó, J. Rebek, Jr., Chem. Commun. 2010, 46, 8630–8632.
- 28The stack plots of the β-CH2 peak of 1 and (1⋅HCl)n at different concentrations and the respective natural logarithm of the normalized signal decay of these peaks are shown in the Supporting Information (Figure S5 and S6, respectively).
- 29A. Wong, R. Ida, L. Spinder, G. Wu, J. Am. Chem. Soc. 2005, 127, 6990–6998. The model takes into account the effect of the number of repeating units on the diffusion coefficient of cylindrical molecular assemblies that do not interact with each other and is based on M. M. Tirado, C. Lopez Martinez, J. García de La Torre, J. Chem. Phys. 1984, 81, 2047–2052.
- 30The hydrodynamic radius is defined as the radius of an equivalent sphere experiencing the same viscous friction as the (1⋅HCl)n species.
- 31Heteroditopic calix[4]arenes endowed with a ureido functionality have been reported to strongly coordinate through hydrogen bonding to DMSO molecules; see: A. Arduini, E. Brindani, G. Giorgi, A. Pochini, A. Secchi, Tetrahedron 2003, 59, 7587–7594.
- 32F. P. Ballistreri, A. Notti, S. Pappalardo, M. F. Parisi, I. Pisagatti, Org. Lett. 2003, 5, 1071–1074.
- 33J. Lacour, D. Morelda, Chem. Commun. 2009, 7073–7079.
- 34Induced CD signals likely arise as the result of an asymmetric arrangement of the calixarene framework (i.e. clockwise and counter-clockwise orientation of the NH groups of the ureido moiety, with respect to the macrocyclic cavity) induced by the binding of the enantiomeric mandelate counterions. An additional helical arrangement (instilled by steric hindrance during the monomer-to-monomer self-assembly process) may also be invoked in the case of the ((S)-MaH⋅1)n and ((R)-MaH⋅1)n assemblies to justify the enhanced intensity of their CD bands.
- 35The low-intensity CD signals, observed in Figure 4 c (traces 2 and 4), account for the CD spectra of (S)- and (R)-mandelic acid, respectively, released in solution upon the addition of trifluoroacetic acid (TFA; see the Supporting Information, Figure S11).
- 36For representative examples, see:
- 36aY. Furusho, T. Kimura, Y. Mizuno, T. Aida, J. Am. Chem. Soc. 1997, 119, 5267–5268;
- 36bL. J. Prins, F. De Jong, P. Timmerman, D. N. Reinhoudt, Nature 2000, 408, 181–184;
- 36cY. Mizuno, T. Aida, K. Yamaguchi, J. Am. Chem. Soc. 2000, 122, 5278–5285;
- 36dJ. M. Rivera, S. L. Craig, T. Martin, J. Rebek, Jr., Angew. Chem. 2000, 112, 2214–2216;
Angew. Chem. Int. Ed. 2000, 39, 2130–2132;
10.1002/1521-3773(20000616)39:12<2130::AID-ANIE2130>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 36eH. Onouchi, T. Miyagawa, K. Morino, E. Yashima, Angew. Chem. 2006, 118, 2441–2444;
10.1002/ange.200504162 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2381–2384;
- 36fA. Mammana, A. D’Urso, R. Lauceri, R. Purrello, J. Am. Chem. Soc. 2007, 129, 8062–8063;
- 36gR. Randazzo, A. Mammana, A. D’Urso, R. Lauceri, R. Purrello, Angew. Chem. 2008, 120, 10027–10030;
10.1002/ange.200803619 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9879–9882;
- 36hF. Helmich, C. C. Lee, A. P. H. J. Schenning, E. W. Meijer, J. Am. Chem. Soc. 2010, 132, 16753–16755.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.