Conjugate Addition/Cyclization Sequence Enables Selective and Simultaneous Fluorescence Detection of Cysteine and Homocysteine†
Corresponding Author
Dr. Xiaofeng Yang
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Institute of Analytical Sciences, College of Chemistry & Materials Science, Northwest University, Xi'an 710069 (P.R. China)
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-stronginSearch for more papers by this authorYixing Guo
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Search for more papers by this authorCorresponding Author
Prof. Robert M. Strongin
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-stronginSearch for more papers by this authorCorresponding Author
Dr. Xiaofeng Yang
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Institute of Analytical Sciences, College of Chemistry & Materials Science, Northwest University, Xi'an 710069 (P.R. China)
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-stronginSearch for more papers by this authorYixing Guo
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Search for more papers by this authorCorresponding Author
Prof. Robert M. Strongin
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-strongin
Department of Chemistry, Portland State University, 1719 SW 10 Ave, Portland, OR 97201 (USA) http://www.pdx.edu/chem/profile/dr-robert-stronginSearch for more papers by this authorSupport from the National Institutes of Health through award RO1 EB002044 is gratefully acknowledged. X.F.Y. acknowledges financial support from the China Scholarship Council.
Graphical Abstract
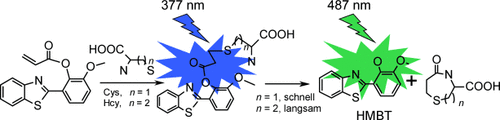
Ähnlich, doch nicht gleich: Ein Benzothiazolderivat kann Cystein (Cys) und Homocystein (Hcy) parallel in neutralen Medien nachweisen. Bei dieser Methode wird zunächst ein Thioether gebildet, bevor eine Lactamcyclisierung unter Freisetzung von 2-(2′-Hydroxy-3′-methoxyphenyl)benzothiazol (HMBT) erfolgt. Der unterschiedlich schnelle Ringschluss ermöglicht die Identifizierung von Cys und Hcy auf spektralem oder kinetischem Weg.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201103759_sm_miscellaneous_information.pdf888.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aZ. A. Wood, E. Schröder, J. R. Harris, L. B. Poole, Trends Biochem. Sci. 2003, 28, 32–40;
- 1bJ. Schulz, J. Lindenau, J. Seyfried, J. Dichgans, Eur. J. Biochem. 2000, 267, 4904–4911.
- 2X. F. Wang, M. S. Cynader, J. Neurosci. 2001, 21, 3322–3331.
- 3S. Shahrokhian, Anal. Chem. 2001, 73, 5972–5978.
- 4aH. Refsum, P. M. Ueland, O. Nygård, S. E. Vollset, Annu. Rev. Med. 1998, 49, 31–62;
- 4bS. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D’Agostino, P. W. F. Wilson, P. A. Wolf, N. Engl. J. Med. 2002, 346, 476–483;
- 4cH. Refsum, A. D. Smith, P. M. Ueland, E. Nexo, R. Clarke, J. McPartlin, C. Johnston, F. Engbaek, J. Schneede, C. McPartlin, J. M. Scott, Clin. Chem. 2004, 50, 3–32.
- 5
- 5aF.-J. Huo, Y.-Q. Sun, J. Su, J.-B. Chao, H.-J. Zhi, C.-X. Yin, Org. Lett. 2009, 11, 4918–4921;
- 5bY. Zeng, G. X. Zhang, D. Q. Zhang, Anal. Chim. Acta 2008, 627, 254–257.
- 6
- 6aH. L. Chen, Q. Zhao, Y. B. Wu, F. Y. Li, H. Yang, T. Yi, C. H. Huang, Inorg. Chem. 2007, 46, 11075–11081;
- 6bS. M. Ji, H. M. Guo, X. L. Yuan, X. H. Li, H. D. Ding, P. Gao, C. X. Zhao, W. T. Wu, W. H. Wu, J. Z. Zhao, Org. Lett. 2010, 12, 2876–2879.
- 7
- 7aX. Q. Chen, Y. Zhou, X. J. Peng, J. Yoon, Chem. Soc. Rev. 2010, 39, 2120–2135;
- 7bZ. Y. Yao, X. L. Feng, C. Li, G. Q. Shi, Chem. Commun. 2009, 5886–5888;
- 7cJ. H. Lee, C. S. Lim, Y. S. Tian, J. H. Han, B. R. Cho, J. Am. Chem. Soc. 2010, 132, 1216–1217;
- 7dB. C. Zhu, X. L. Zhang, Y. M. Li, P. F. Wang, H. Y. Zhang, X. Q. Zhuang, Chem. Commun. 2010, 46, 5710–5712.
- 8
- 8aH. Kwon, K. Lee, H. J. Kim, Chem. Commun. 2011, 47, 1773–1775;
- 8bH. S. Jung, K. C. Ko, G. H. Kim, A. R. Lee, Y. C. Na, C. Kang, J. Y. Lee, J. S. Kim, Org. Lett. 2011, 13, 1498–1501;
- 8cY. Liu, Y. Yu, J. W. Y. Lam, Y. N. Hong, M. Faisal, W. Z. Yuan, B. Z. Tang, Chem. Eur. J. 2010, 16, 8433–8438;
- 8dW. Jiang, Q. Q. Fu, H. Y. Fan, J. Ho, W. Wang, Angew. Chem. 2007, 119, 8597–8600; Angew. Chem. Int. Ed. 2007, 46, 8445–8448;
- 8eL. Yi, H. Y. Li, L. Sun, L. L. Liu, C. H. Zhang, Z. Xi, Angew. Chem. 2009, 121, 4094–4097; Angew. Chem. Int. Ed. 2009, 48, 4034–4037;
- 8fW. Y. Lin, L. Yuan, Z. M. Cao, Y. M. Feng, L. L. Long, Chem. Eur. J. 2009, 15, 5096–5103;
- 8gT. Matsumoto, Y. Urano, T. Shoda, H. Kojima, T. Nagano, Org. Lett. 2007, 9, 3375–3377;
- 8hX.-F. Guo, H. Wang, Y.-H. Guo, H.-S. Zhang, Anal. Chim. Acta 2009, 633, 71–75;
- 8iX. Q. Chen, S.-K. Ko, M. J. Kim, I. Shin, J. Yoon, Chem. Commun. 2010, 46, 2751–2753;
- 8jS. Sreejith, K. P. Divya, A. Ajayaghosh, Angew. Chem. 2008, 120, 8001–8005;
10.1002/ange.200803194 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7883–7887;
- 8kV. Hong, A. A. Kislukhin, M. G. Finn, J. Am. Chem. Soc. 2009, 131, 9986–9994.
- 9
- 9aH. Y. Shiu, H. C. Chong, Y. C. Leung, M. K. Wong, C. M. Che, Chem. Eur. J. 2010, 16, 3308–3313;
- 9bL. L. Long, W. Y. Lin, B. B. Chen, W. S. Gao, L. Yuan, Chem. Commun. 2011, 47, 893–895;
- 9cJ. Y. Shao, H. M. Guo, S. M. Ji, J. Z. Zhao, Biosens. Bioelectron. 2011, 26, 3012–3017;
- 9dX. Li, S. J. Qian, Q. J. He, B. Yang, J. Li, Y. Z. Hu, Org. Biomol. Chem. 2010, 8, 3627–3630;
- 9eH. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki, N. Itoh, Angew. Chem. 2005, 117, 2982–2985; Angew. Chem. Int. Ed. 2005, 44, 2922–2925; Angew. Chem. Int. Ed. 2005, 44, 2922–2925;
- 9fS.-P. Wang, W.-J. Deng, D. Sun, M. Yan, H. Zheng, J.-G. Xu, Org. Biomol. Chem. 2009, 7, 4017–4020;
- 9gJ. Bouffard, Y. Kim, T. M. Swager, R. Weissleder, S. A. Hilderbrand, Org. Lett. 2008, 10, 37–40;
- 9hW. Jiang, Q. Q. Fu, H. Y. Fan, J. Ho, W. Wang, Angew. Chem. 2007, 119, 8597–8600; Angew. Chem. Int. Ed. 2007, 46, 8445–8448; Angew. Chem. Int. Ed. 2007, 46, 8445–8448;
- 9iA. Shibata, K. Furukawa, H. Abe, S. Tsuneda, Y. Ito, Bioorg. Med. Chem. Lett. 2008, 18, 2246–2249;
- 9jB. Tang, L. L Yin, X. Wang, Z. Z. Chen, L. L. Tong, K. H. Xu, Chem. Commun. 2009, 5293–5295;
- 9kB. Tang, Y. L. Xing, P. Li, N. Zhang, F. B. Yu, G. W. Yang, J. Am. Chem. Soc. 2007, 129, 11666–11667;
- 9lX.-F. Yang, Z. Su, C. H. Liu, H. P. Qi, M. L. Zhao, Anal. Bioanal. Chem. 2010, 396, 2667–2674.
- 10
- 10aM. Zhang, M. X. Yu, F. Y. Li, M. W. Zhu, M. Y. Li, Y. H. Gao, L. Li, Z. Q. Liu, J. P. Zhang, D. Q. Zhang, T. Yi, C. H. Huang, J. Am. Chem. Soc. 2007, 129, 10322–10323;
- 10bS.-T. Huang, K.-N. Ting, K.-L. Wang, Anal. Chim. Acta 2008, 620, 120–126;
- 10cRef. [7c];
- 10dM. M. Pires, J. Chmielewski, Org. lett. 2008, 10, 837–840;
- 10eY.-K. Yang, S. Shim, J. Tae, Chem. Commun. 2010, 46, 7766–7768.
- 11
- 11aO. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian, R. M. Strongin, J. Am. Chem. Soc. 2004, 126, 438–439;
- 11bW. H. Wang, O. Rusin, X. Y. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner, R. M. Strongin, J. Am. Chem. Soc. 2005, 127, 15949–15958.
- 12
- 12aH. L. Li, J. L. Fan, J. Y. Wang, M. Z. Tian, J. J. Du, S. G. Sun, P. P. Sun, X. J. Peng, Chem. Commun. 2009, 5904–5906;
- 12bK.-S. Lee, T.-K. Kim, J. H. Lee, H.-J. Kim, J.-I. Hong, Chem. Commun. 2008, 6173–6175;
- 12cF. Tanaka, N. Mase, C. F. Barbas III, Chem. Commun. 2004, 1762–1763;
- 12dRef. [12a];
- 12eL. P. Duan, Y. F. Xu, X. H. Qian, F. Wang, J. W. Liu, T. Y. Cheng, Tetrahedron Lett. 2008, 49, 6624–6627;
- 12fT.-K. Kim, D.-N. Lee, H.-J. Kim, Tetrahedron Lett. 2008, 49, 4879–4881;
- 12gM. Zhang, M. Y. Li, Q. Zhao, F. Y. Li, D. Q. Zhang, J. P. Zhang, T. Yi, C. H. Huang, Tetrahedron Lett. 2007, 48, 2329–2333;
- 12hX. J. Zhang, X. S. Ren, Q.-H. Xu, K. P. Loh, Z.-K. Chen, Org. Lett. 2009, 11, 1257–1260;
- 12iS. Lim, J. O. Escobedo, M. Lowry, X. Y. Xu, R. Strongin, Chem. Commun. 2010, 46, 5707–5709.
- 13
- 13aP. Blondeau, R. Gauthier, C. Berse, D. Gravel, Can. J. Chem. 1971, 49, 3866–3876;
- 13bN. J. Leonard, R. Y. Ning, J. Org. Chem. 1966, 31, 3928–3935.
- 14G. L. Khatik, R. Kumar, A. K. Chakraborti, Org. Lett. 2006, 8, 2433–2436.
- 15
- 15aG. Illuminati, L. Mandolini, Acc. Chem. Res. 1981, 14, 95–102;
- 15bC. Galli, L. Mandolini, Eur. J. Org. Chem. 2000, 3117–3125;
- 15cC. Galli, G. Illuminati, L. Mandolini, P. Tamborra, J. Am. Chem. Soc. 1977, 99, 2591–2597.
- 16
- 16aS. Lochbrunner, A. J. Wurzer, E. Riedlea, J. Chem. Phys. 2000, 112, 10699–10702;
- 16bW. E. Brewer, M. L. Martinez, P.-T. Chou, J. Phys. Chem. 1990, 94, 1915–1918.
- 17
- 17aR. Hu, J. Feng, D. H. Hu, S. Q. Wang, S. Y. Li, Y. Li, G. Q. Yang, Angew. Chem. 2010, 122, 5035–5038; Angew. Chem. Int. Ed. 2010, 49, 4915–4918;
- 17bX.-F. Yang, H. P. Qi, L. P. Wang, Z. Su, G. Wang, Talanta 2009, 80, 92–97;
- 17cT.-I. Kim, H. J. Kang, G. Han, S. J. Chung, Y. Kim, Chem. Commun. 2009, 5895–5897.
- 18Total Hcy concentration in healthy human plasma is approximately 5–12 μM. Cys concentration is approximately 20–30 times that of Hcy (See Ref. [4a,b] and [11a]).
- 19R. Carmel, D. W. Jacobsen in Homocysteine in Health and Disease, Vol. 28 (Eds.: ), Cambridge University Press, Cambridge, 2001, pp. 341–355.
- 20G. Sharma, R. Kumar, A. K. Chakraborti, Tetrahedron Lett. 2008, 49, 4269–4271.
- 21The human plasma samples were deproteinized by using the procedure as described in the literature: J. G. Ma, G. Stoter, J. Verweij, J. H. M. Schellens, Cancer Chemother. Pharmacol. 1996, 38, 391–394.
- 22E. Beutler, T. Gelbart, J. Lab. Clin. Med. 1985, 105, 581–584.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.