OsO4⋅Streptavidin: A Tunable Hybrid Catalyst for the Enantioselective cis-Dihydroxylation of Olefins†
Dr. Valentin Köhler
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDr. Jincheng Mao
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorTillmann Heinisch
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDr. Anca Pordea
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Alessia Sardo
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Yvonne M. Wilson
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorLivia Knörr
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Marc Creus
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorJean-Christophe Prost
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Tilman Schirmer
Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Tilman Schirmer, Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Thomas R. Ward, Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas R. Ward
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Tilman Schirmer, Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Thomas R. Ward, Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Valentin Köhler
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDr. Jincheng Mao
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorTillmann Heinisch
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDr. Anca Pordea
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Alessia Sardo
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Yvonne M. Wilson
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorLivia Knörr
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Marc Creus
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorJean-Christophe Prost
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Tilman Schirmer
Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Tilman Schirmer, Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Thomas R. Ward, Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas R. Ward
Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Tilman Schirmer, Biozentrum, Universität Basel, Klingelbergstrasse 50/70, 4056 Basel (Switzerland)
Thomas R. Ward, Departement Chemie, Universität Basel, Spitalstrasse 51, 4056 Basel (Switzerland)
Search for more papers by this authorWe thank Prof. C. R. Cantor for the SAV gene, Chiral Technologies Europe for help with the method development for chiral-phase HPLC analysis, Beat Amrein for preliminary experiments, and Maurus Schmid for his contribution to the artwork. This research was funded by the Swiss National Science Foundation (Grant 200020-126366), the Cantons of Basel, and the Marie Curie Training Network (FP7-ITN-238434).
Graphical Abstract
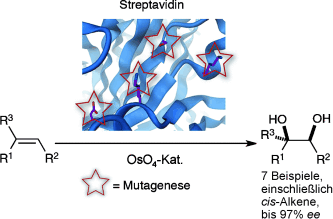
Gut kombiniert: Die Kombination von Apostreptavidin mit OsO4 lieferte selektive Katalysatoren für die Olefindihydroxylierung. Durch ortsgerichtete Mutagenese gelang eine Verbesserung der Enantioselektivität und in bestimmten Fällen sogar eine Umkehr der Enantiopräferenz. Vor allem Allylphenylsulfid und cis-β-Methylstyrol wurden mit bislang unerreichtem Enantiomerenüberschuss umgesetzt.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201103632_sm_miscellaneous_information.pdf1.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Lu, N. Yeung, N. Sieracki, N. M. Marshall, Nature 2009, 460, 855–862;
- 1bF. Rosati, G. Roelfes, ChemCatChem 2010, 2, 916–927;
- 1cM. T. Reetz, Top. Organomet. Chem. 2009, 25, 63–92;
- 1dS. Abe, T. Ueno, Y. Watanabe, Top. Organomet. Chem. 2009, 25, 25–43;
- 1eQ. Jing, R. J. Kazlauskas, ChemCatChem 2010, 2, 953–957;
- 1fP. J. Deuss, G. Popa, C. H. Botting, W. Laan, P. C. Kamer, Angew. Chem. 2010, 122, 5443–5445;
10.1002/ange.201002174 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5315–5317;
- 1gJ. Podtetenieff, A. Taglieber, E. Bill, E. J. Reijerse, M. T. Reetz, Angew. Chem. 2010, 122, 5277–5281;
10.1002/ange.201002106 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5151–5155;
- 1hT. Heinisch, T. R. Ward, Curr. Opin. Chem. Biol. 2010, 14, 184–199;
- 1iP. Fournier, R. Fiammengo, A. Jäschke, Angew. Chem. 2009, 121, 4490–4493; Angew. Chem. Int. Ed. 2009, 48, 4426–4429.
- 2
- 2aH. C. Kolb, M. S. VanNieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483–2587;
- 2bT. Oishi, M. Hirama, Tetrahedron Lett. 1992, 33, 639–642;
- 2cY. Imada, T. Saito, T. Kawakami, S.-I. Murahashi, Tetrahedron Lett. 1992, 33, 5081–5084;
- 2dT. Kokubo, T. Sugimoto, T. Uchida, S. Tanimoto, M. Okano, J. Chem. Soc. Chem. Commun. 1983, 769–770;
- 2eM. A. Andersson, R. Epple, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 490–493;
10.1002/1521-3757(20020201)114:3<490::AID-ANGE490>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2002, 41, 472–475;10.1002/1521-3773(20020201)41:3<472::AID-ANIE472>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 2fA. B. Zaitsev, H. Adolfsson, Synthesis 2006, 1725–1756;
- 2gK. B. Sharpless, Angew. Chem. 2002, 114, 2126–2135;
Angew. Chem. Int. Ed. 2002, 41, 2024–2032;
10.1002/1521-3773(20020617)41:12<2024::AID-ANIE2024>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 2hZ.-M. Wang, X.-L. Zhang, K. B. Sharpless, Tetrahedron Lett. 1993, 34, 2267–2270;
- 2iP. J. Walsh, P. T. Ho, S. B. King, K. B. Sharpless, Tetrahedron Lett. 1994, 35, 5129–5132;
- 2jH. Becker, S. B. King, M. Taniguchi, K. P. M. Vanhessche, K. B. Sharpless, J. Org. Chem. 1995, 60, 3940–3941;
- 2kL. Wang, K. B. Sharpless, J. Am. Chem. Soc. 1992, 114, 7568–7570;
- 2lZ.-M. Wang, K. Kakiuchi, K. B. Sharpless, J. Org. Chem. 1994, 59, 6895–6897;
- 2mH. C. Kolb, P. G. Andersson, K. B. Sharpless, J. Am. Chem. Soc. 1994, 116, 1278–1291;
- 2nS. Y. Jonsson, K. Färnegårdh, J.-E. Bäckvall, J. Am. Chem. Soc. 2001, 123, 1365–1371;
- 2oK. Bergstad, S. Y. Jonsson, J.-E. Bäckvall, J. Am. Chem. Soc. 1999, 121, 10424–10425.
- 3
- 3aD. R. Boyd, G. N. Sheldrake, Nat. Prod. Rep. 1998, 15, 309–324;
- 3bT. Hudlicky, D. Gonzalez, D. T. Gibson, Aldrichimica Acta 1999, 32, 35–62;
- 3cA. Karlsson, J. V. Parales, R. E. Parales, D. T. Gibson, H. Eklund, S. Ramaswamy, Science 2003, 299, 1039–1042;
- 3dT. Hudlicky, J. W. Reed, Synlett 2009, 685–703.
- 4P. A. Colussi, T. C. Evans, C. H. Taron (New England Biolabs, Inc.), WO2007123689, 2007.
- 5N. Humbert, A. Zocchi, T. R. Ward, Electrophoresis 2005, 26, 47–52.
- 6Although the effect of mutating K132 has not yet been investigated, the results obtained with mutations at other sites (namely S112, K121, L124, and D128) render the involvement of this amino acid side chain unlikely.
- 7The performance of mutants/batches used in this study was first evaluated with various ratios of osmate to protein to determine the loading that led to the highest HPLC yield without erosion of enantioselectivity; see the Supporting Information for details.
- 8
- 8aP. C. Weber, D. H. Ohlendorf, J. J. Wendoloski, F. R. Salemme, Science 1989, 243, 85–88;
- 8bP. C. Weber, J. J. Wendoloski, M. W. Pantoliano, F. R. Salemme, J. Am. Chem. Soc. 1992, 114, 3197–3200;
- 8cJ. DeChancie, K. N. Houk, J. Am. Chem. Soc. 2007, 129, 5419–5429;
- 8dS. Freitag, V. Chu, J. E. Penzotti, L. A. Klumb, R. To, D. Hyre, I. Le Trong, T. P. Lybrand, R. E. Stenkamp, P. S. Stayton, Proc. Natl. Acad. Sci. USA 1999, 96, 8384–8389.
- 9A. Pordea, M. Creus, J. Panek, C. Duboc, D. Mathis, M. Novic, T. R. Ward, J. Am. Chem. Soc. 2008, 130, 8085–8088.
- 10The residue K121 is significantly closer to residue D128 of the adjacent monomer in the tetrameric structure of SAV than to the D128 on the same oligopeptide chain (12.67 vs. 20.70 Å for Cα).
- 11After an initial screening of a wider range of SAV mutants with styrene, five SAV S112X mutants (S112N, S112A, S112T, S112M, S112Y), four SAV K121X (K121A, K121F, K121N, K121H), and four SAV L124X mutants (L124G, L124K, L124N, L124Y) were selected for further study.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.