Dinuclear Palladium(III) Complexes with a Single Unsupported Bridging Halide Ligand: Reversible Formation from Mononuclear Palladium(II) or Palladium(IV) Precursors†
Dr. Julia R. Khusnutdinova
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481
Search for more papers by this authorDr. Nigam P. Rath
Department of Chemistry and Biochemistry, University of Missouri–St. Louis (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Liviu M. Mirica
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481Search for more papers by this authorDr. Julia R. Khusnutdinova
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481
Search for more papers by this authorDr. Nigam P. Rath
Department of Chemistry and Biochemistry, University of Missouri–St. Louis (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Liviu M. Mirica
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481
Department of Chemistry, Washington University, St. Louis, MO 63130 (USA), Fax: (+1) 314-935-4481Search for more papers by this authorWe thank the Department of Chemistry at Washington University and American Chemical Society Petroleum Research Fund (49914-DNI3) for support. We also thank Prof. T. Daniel P. Stack for a gift of the ligand Me3tacn.
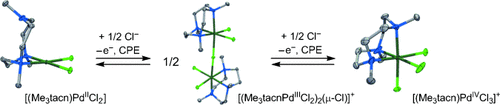
Graphical Abstract
Stabiles PdIII: Zweikernige PdIII-Komplexe des dreizähnigen Liganden Trimethyltriazacyclononan (Me3tacn) wurden durch Einelektronenoxidation einkerniger PdII-Vorstufen erhalten. Die weitere Oxidation ergab reversibel einkernige PdIV-Spezies. Die PdII- und PdIII-Komplexe spielen in der katalytischen Kharasch-Addition von Polyhalogenalkanen an Alkene eine Rolle.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100928_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, Hoboken, NJ, 2002.
10.1002/0471212466 Google Scholar
- 2
- 2aK. Muñiz, Angew. Chem. 2009, 121, 9576;
10.1002/ange.200903671 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9412;
- 2bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 2cA. J. Canty, Dalton Trans. 2009, 10409;
- 2dP. Sehnal, R. J. K. Taylor, I. J. S. Fairlamb, Chem. Rev. 2010, 110, 824.
- 3
- 3aD. C. Powers, T. Ritter, Nat. Chem. 2009, 1, 302;
- 3bD. C. Powers, M. A. L. Geibel, J. Klein, T. Ritter, J. Am. Chem. Soc. 2009, 131, 17050.
- 4N. R. Deprez, M. S. Sanford, J. Am. Chem. Soc. 2009, 131, 11234.
- 5M. P. Lanci, M. S. Remy, W. Kaminsky, J. M. Mayer, M. S. Sanford, J. Am. Chem. Soc. 2009, 131, 15618.
- 6J. R. Khusnutdinova, N. P. Rath, L. M. Mirica, J. Am. Chem. Soc. 2010, 132, 7303.
- 7
- 7aF. A. Cotton, I. O. Koshevoy, P. Lahuerta, C. A. Murillo, M. Sanau, M. A. Ubeda, Q. Zhao, J. Am. Chem. Soc. 2006, 128, 13674;
- 7bF. A. Cotton, J. D. Gu, C. A. Murillo, D. J. Timmons, J. Am. Chem. Soc. 1998, 120, 13280.
- 8
- 8aS. Takaishi, M. Takamura, T. Kajiwara, H. Miyasaka, M. Yamashita, M. Lwata, H. Matsuzaki, H. Okarnoto, H. Tanaka, S. Kuroda, H. Nishikawa, H. Oshio, K. Kato, M. Takata, J. Am. Chem. Soc. 2008, 130, 12080;
- 8bM. Yamashita, S. Takaishi, Chem. Commun. 2010, 46, 4438.
- 9
- 9aA. J. Blake, L. M. Gordon, A. J. Holder, T. I. Hyde, G. Reid, M. Schröder, J. Chem. Soc. Chem. Commun. 1988, 1452;
- 9bG. Hunter, A. McAuley, T. W. Whitcombe, Inorg. Chem. 1988, 27, 2634;
- 9cA. McAuley, T. W. Whitcombe, Inorg. Chem. 1988, 27, 3090;
- 9dT. N. Margulis, L. J. Zompa, Inorg. Chim. Acta 1992, 201, 61;
- 9eA. A. Sobanov, A. N. Vedernikov, G. Dyker, B. N. Solomonov, Mendeleev Commun. 2002, 12, 14.
- 10A. J. Blake, A. J. Holder, Y. V. Roberts, M. Schröder, J. Chem. Soc. Chem. Commun. 1993, 260.
- 11
- 11aK. Wieghardt, E. Schoeffmann, B. Nuber, J. Weiss, Inorg. Chem. 1986, 25, 4877;
- 11bO. Schlager, K. Wieghardt, B. Nuber, Inorg. Chem. 1995, 34, 6449;
- 11cA. J. Blake, I. A. Fallis, A. Heppeler, S. Parsons, S. A. Ross, M. Schröder, J. Chem. Soc. Dalton Trans. 1996, 31;
- 11dA. J. Blake, I. A. Fallis, S. Parsons, S. A. Ross, M. Schröder, J. Chem. Soc. Dalton Trans. 1996, 525.
- 12See the Supporting Information.
- 13CCDC 809190 (1 a), 809186 (2 a, T=100 K), 809187 (2 a, T=293 K), 809183 (2 b), 809185 (2 c), 809188 (3 a), 809189 (3 b), and 809184 ([(Me3tacn)PdIICl(MeCN)]ClO4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14Coulometry measurements confirm that both oxidations correspond to one electron processes (see the Supporting Information).
- 15The presence of dimers 2 a and 2 b in solution was confirmed by ESI-MS and the lack of an EPR signal for the corresponding solutions at 77 K or RT.
- 16The presence of [(Me3tacn)PdCl(MeCN)]+ in solution after one-electron oxidation of 1 a was detected by CV and confirmed by independent synthesis (see the Supporting Information). If (Me3tacn)PdCl(MeCN)+ is the only halide abstraction product, then the theoretical yield of 2 a is 67 %.
- 17The high extinction coefficients of 2 a,b enable the unique visualization of mass-transport processes at the disk electrode during CV of 1 a,b (Supporting Information, Figure S15).
- 18J. Terheijden, G. van Koten, D. M. Grove, K. Vrieze, A. L. Spek, J. Chem. Soc. Dalton Trans. 1987, 1359.
- 19
- 19aK. Toriumi, Y. Wada, T. Mitani, S. Bandow, M. Yamashita, Y. Fujii, J. Am. Chem. Soc. 1989, 111, 2341;
- 19bM. Yamashita, T. Manabe, K. Inoue, T. Kawashima, H. Okamoto, H. Kitagawa, T. Mitani, K. Toriumi, H. Miyamae, R. Ikeda, Inorg. Chem. 1999, 38, 1894;
- 19cS. Takaishi, H. S. Wu, J. X. Xie, T. Kajiwara, B. K. Breedlove, H. Miyasaka, M. Yamashita, Inorg. Chem. 2010, 49, 3694;
- 19dM. Yamashita, T. Ishii, H. Matsuzaka, T. Manabe, T. Kawashima, H. Okamoto, H. Kitagawa, T. Mitani, K. Marumoto, S. Kuroda, Inorg. Chem. 1999, 38, 5124.
- 20M. B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1968, 10, 247.
- 21No positional disorder along the Pd(μ-X)Pd axis was found for the bridging halide in the structures of either 2 a (both at RT and 100 K) or 2 b,c (see the Supporting Information).
- 22For example, the Pd⋅⋅⋅Pd distance of the only known PdIIIBrPdIII 1D chain is 5.21 Å at 110 K (Ref. [8a]).
- 23SQUID measurements of 2 a showed a negligible magnetic susceptibility in the 2–320 K range.
- 24No other absorption bands were seen in the range 700–2200 nm.
- 25B. S. Brunschwig, C. Creutz, N. Sutin, Chem. Soc. Rev. 2002, 31, 168.
- 26Although the α HOMO is mainly localized on Pd1 whereas the α LUMO+1 is mainly localized on Pd2, both α→α and β→β transitions have identical contributions to the TD-DFT calculated absorption bands (see the Supporting Information). Similar MMCT transitions have been proposed to exist in antiferromagnetically coupled dinuclear CuII complexes: F. Tuczek, E. I. Solomon, Coord. Chem. Rev. 2001, 219, 1075.
- 27Only a limited number of cationic PdIV complexes have been structurally characterized to date:
- 27aK. Toriumi, M. Yamashita, H. Ito, T. Ito, Acta Crystallogr. Sect. C 1986, 42, 963;
- 27bP. K. Byers, A. J. Canty, B. W. Skelton, A. H. White, J. Chem. Soc. Chem. Commun. 1987, 1093;
- 27cP. K. Byers, A. J. Canty, B. W. Skelton, A. H. White, Organometallics 1990, 9, 826;
- 27dA. Bayler, A. J. Canty, P. G. Edwards, B. W. Skelton, A. H. White, J. Chem. Soc. Dalton Trans. 2000, 3325;
- 27eA. J. Canty, M. J. G. Hettinga, J. Patel, M. Pfeffer, B. W. Skelton, A. H. White, Inorg. Chim. Acta 2002, 338, 94;
- 27fW. Oloo, P. Y. Zavalij, J. Zhang, E. Khaskin, A. N. Vedernikov, J. Am. Chem. Soc. 2010, 132, 14400.
- 28
- 28aR. A. Gossage, L. A. van de Kuil, G. van Koten, Acc. Chem. Res. 1998, 31, 423;
- 28bL. A. van de Kuil, D. M. Grove, R. A. Gossage, J. W. Zwikker, L. W. Jenneskens, W. Drenth, G. van Koten, Organometallics 1997, 16, 4985.
- 29While other PdII complexes have been reported as catalysts for the Kharasch reaction, no PdIII intermediates have been detected to date:
- 29aJ. Tsuji, K. Sato, H. Nagashima, Tetrahedron 1985, 41, 393;
- 29bD. Motoda, H. Kinoshita, H. Shinokubo, K. Oshima, Adv. Synth. Catal. 2002, 344, 261;
- 29cA. S. Dneprovskii, A. A. Ermoshkin, A. N. Kasatochkin, V. P. Boyarskii, Russ. J. Org. Chem. 2003, 39, 933.
- 30P. Neta, J. Grodkowski, A. B. Ross, J. Phys. Chem. Ref. Data 1996, 25, 709.
- 31Chemical oxidation of 1 b with 0.5 equiv Br2 generates 2 b in 40 % yield (see the Supporting Information). No PdIV complexes were detected during chemical oxidation with excess Br2 or CCl3Br.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.