Racemase Activity of B. cepacia Lipase Leads to Dual-Function Asymmetric Dynamic Kinetic Resolution of α-Aminonitriles†
Dr. Pornrapee Vongvilai
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Search for more papers by this authorMats Linder
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
These authors contributed equally.
Search for more papers by this authorMorakot Sakulsombat
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
These authors contributed equally.
Search for more papers by this authorDr. Maria Svedendahl Humble
School of Biotechnology, KTH—Royal Institute of Technology, AlbaNova University Center, 106 91 Stockholm (Sweden)
Search for more papers by this authorProf. Per Berglund
School of Biotechnology, KTH—Royal Institute of Technology, AlbaNova University Center, 106 91 Stockholm (Sweden)
Search for more papers by this authorProf. Tore Brinck
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Search for more papers by this authorCorresponding Author
Prof. Olof Ramström
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333Search for more papers by this authorDr. Pornrapee Vongvilai
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Search for more papers by this authorMats Linder
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
These authors contributed equally.
Search for more papers by this authorMorakot Sakulsombat
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
These authors contributed equally.
Search for more papers by this authorDr. Maria Svedendahl Humble
School of Biotechnology, KTH—Royal Institute of Technology, AlbaNova University Center, 106 91 Stockholm (Sweden)
Search for more papers by this authorProf. Per Berglund
School of Biotechnology, KTH—Royal Institute of Technology, AlbaNova University Center, 106 91 Stockholm (Sweden)
Search for more papers by this authorProf. Tore Brinck
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Search for more papers by this authorCorresponding Author
Prof. Olof Ramström
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333
Department of Chemistry, KTH—Royal Institute of Technology, Teknikringen 30, 10044 Stockholm (Sweden), Fax: (+46) 8-791-2333Search for more papers by this authorThis study was supported by the Swedish Research Council and the Royal Institute of Technology. We thank Toyo Denka Kogyo Co. and Dr. Y. Yamashita for providing samples for this study.
Graphical Abstract
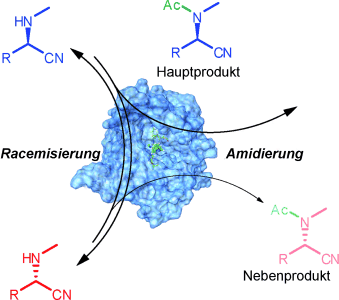
Begrüßenswerte Promiskuität: Die Racemase-artige Aktivität von B.-cepacia-Lipase gegenüber N-substituierten α-Aminonitrilen sollte nach experimentellen Befunden und Rechnungen auf einer C-C-Spaltung/Verknüpfung im Hydrolasezentrum des Enzyms beruhen. Diese promiskuitive Aktivität ermöglicht in Kombination mit der Transacylierungsaktivität des Enzyms die asymmetrische Synthese von N-Methyl-α-aminonitrilamiden in hoher Ausbeute (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201007373_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Noyori, Nat. Chem. 2009, 1, 5–6.
- 2M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Keßeler, R. Stürmer, T. Zelinski, Angew. Chem. 2004, 116, 806–843;
10.1002/ange.200300599 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 788–824.
- 3A. Ghanem, Tetrahedron 2007, 63, 1721–1754.
- 4H. E. Schoemaker, D. Mink, M. G. Wubbolts, Science 2003, 299, 1694–1697.
- 5For recent accounts, see:
- 5aD. Liu, P. Trodler, S. Eiben, K. Koschorreck, M. Müller, J. Pleiss, S. C. Maurer, C. Branneby, R. D. Schmid, B. Hauer, ChemBioChem 2010, 11, 789–795;
- 5bN. Ríos-Lombardía, E. Busto, V. Gotor-Fernández, V. Gotor, R. Porcar, E. García-Verdugo, S. V. Luis, I. Alfonso, S. García-Granda, A. Menéndez-Velázquez, Chem. Eur. J. 2010, 16, 836–847;
- 5cM. Vallin, P.-O. Syrén, K. Hult, ChemBioChem 2010, 11, 411–416;
- 5dT. Ema, S. Kamata, M. Takeda, Y. Nakano, T. Sakai, Chem. Commun. 2010, 46, 5440–5442;
- 5eS. Akai, R. Hanada, N. Fujiwara, Y. Kita, M. Egi, Org. Lett. 2010, 12, 4900–4903;
- 5fL. H. Andrade, T. Barcellos, Org. Lett. 2009, 11, 3052–3055;
- 5gR. O. de Souza, O. A. C. Antunes, W. Kroutil, C. O. Kappe, J. Org. Chem. 2009, 74, 6157–6162;
- 5hP. Vongvilai, R. Larsson, O. Ramström, Adv. Synth. Catal. 2008, 350, 448–452;
- 5iM.-J. Kim, Y. K. Choi, S. Kim, D. Kim, K. Han, S.-B. Ko, J. Park, Org. Lett. 2008, 10, 1295–1298;
- 5jP. Vongvilai, M. Angelin, R. Larsson, O. Ramström, Angew. Chem. 2007, 119, 966–968;
10.1002/ange.200603740 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 948–950;
- 5kN. M. T. Lourenço, C. A. M. Afonso, Angew. Chem. 2007, 119, 8326;
10.1002/ange.200701774 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8178–8181;
- 5lY. Zhu, K.-L. Fow, G.-K. Chuah, S. Jaenicke, Chem. Eur. J. 2007, 13, 541–547;
- 5mB. A. C. van As, J. van Buijtenen, T. Mes, A. R. A. Palmans, E. W. Meijer, Chem. Eur. J. 2007, 13, 8325–8332.
- 6M. Rodríguez-Mata, E. García-Urdiales, V. Gotor-Fernández, V. Gotor, Adv. Synth. Catal. 2010, 352, 395–406.
- 7M. Shakeri, K. Engström, A. G. Sandström, J.-E. Bäckvall, ChemCatChem 2010, 2, 534–538.
- 8L. K. Thalén, D. Zhao, J.-B. Sortais, J. Paetzold, C. Hoben, J.-E. Bäckvall, Chem. Eur. J. 2009, 15, 3403–3410.
- 9V. Gotor-Fernández, E. Busto, V. Gotor, Adv. Synth. Catal. 2006, 348, 797–812.
- 10F. van Rantwijk, R. A. Sheldon, Tetrahedron 2004, 60, 501–519.
- 11G. Asensio, C. Andreu, J. A. Marco, Tetrahedron Lett. 1991, 32, 4197–4198.
- 12T.-W. Chiou, C.-C. Chang, C.-T. Lai, D.-F. Tai, Bioorg. Med. Chem. Lett. 1997, 7, 433–436.
- 13G. F. Breen, Tetrahedron: Asymmetry 2004, 15, 1427–1430.
- 14V. Gotor-Fernández, P. Fernández-Torres, V. Gotor, Tetrahedron: Asymmetry 2006, 17, 2558–2564.
- 15A. J. Blacker, M. J. Stirling, M. I. Page, Org. Process Res. Dev. 2007, 11, 642–648.
- 16A. Liljeblad, H.-M. Kavenius, P. Taehtinen, L. T. Kanerva, Tetrahedron: Asymmetry 2007, 18, 181–191.
- 17P. Vongvilai, O. Ramström, J. Am. Chem. Soc. 2009, 131, 14419–14425.
- 18H. Gröger, Chem. Rev. 2003, 103, 2795–2827.
- 19K. Nakai, J. Hiratake, J. i. Oda, Bull. Inst. Chem. Res. 1992, 70, 333–337.
- 20P. López-Serrano, J. A. Jongejan, F. van Rantwijk, R. A. Sheldon, Tetrahedron: Asymmetry 2001, 12, 219–228.
- 21Racemization effects were observed for cyanoalkyl acetamides when preparations of Candida antarctica lipase B were used in the presence of a zeolite.[20]
- 22U. T. Bornscheuer, R. J. Kazlauskas, Angew. Chem. 2004, 116, 6156–6165; Angew. Chem. Int. Ed. 2004, 43, 6032–6040.
- 23A. Aharoni, L. Gaidukov, O. Khersonsky, S. M. Gould, C. Roodveldt, D. S. Tawfik, Nat. Genet. 2005, 37, 73–76.
- 24K. Hult, P. Berglund, Trends Biotechnol. 2007, 25, 231–238.
- 25H. Pellissier, Tetrahedron 2008, 64, 1563–1601.
- 26A. H. Kamaruddin, M. H. Uzir, H. Y. Aboul-Enein, H. N. A. Halim, Chirality 2009, 21, 449–467.
- 27B. Martín-Matute, J.-E. Bäckvall, Curr. Opin. Chem. Biol. 2007, 11, 226–232.
- 28See the Supporting Information for more details.
- 29
- 29aP. Carlqvist, M. Svedendahl, C. Branneby, K. Hult, T. Brinck, P. Berglund, ChemBioChem 2005, 6, 331–336;
- 29bC. Branneby, P. Carlqvist, A. Magnusson, K. Hult, T. Brinck, P. Berglund, J. Am. Chem. Soc. 2003, 125, 874–875;
- 29cM. Persson, E. Wehtje, P. Adlercreutz, ChemBioChem 2002, 3, 566–571;
10.1002/1439-7633(20020603)3:6<566::AID-CBIC566>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 29dC. Bovet, R. Zenobi, Anal. Biochem. 2008, 373, 380–382;
- 29eD. Rotticci, T. Norin, K. Hult, M. Martinelle, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1483, 132–140.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.