Constructing Multiply Substituted Arenes Using Sequential Palladium(II)-Catalyzed CH Olefination†
Keary M. Engle
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorDong-Hui Wang
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorJin-Quan Yu Prof.
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorKeary M. Engle
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorDong-Hui Wang
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorJin-Quan Yu Prof.
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 N. Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2409 http://www.scripps.edu/chem/yu/index/html
Search for more papers by this authorWe gratefully acknowledge TSRI, the NIH (NIGMS, 1 R01 GM084019-02), Amgen, and Eli Lilly for financial support. We thank the A. P. Sloan Foundation for a fellowship (J.-Q.Y.) and the NSF, the DOD, TSRI, and the Skaggs Oxford Scholarship program for predoctoral fellowships (K.M.E.).
Graphical Abstract
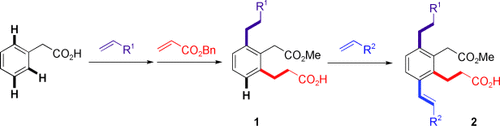
Balanceakt: In den beschriebenen komplementären Katalysesystemen lassen sich Reaktivität und Selektivität bei PdII-katalysierten ortho-C-H-Olefinierungen gegeneinander abwägen, um den schnellen Aufbau der 1,2,3-trisubstituierten Arene 1 durch schrittweise C-H-Funktionalisierung zu ermöglichen. Außerdem gelang eine iterative C-H-Aktivierung so, dass eine neu eingeführte funktionelle Gruppe eine anschließende C-H-Aktivierung steuert (2).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201002077_sm_miscellaneous_information.pdf930.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, Nature 1990, 347, 539.
- 2For reviews of electroluminescent conjugated polymers, see:
- 2aA. Kraft, A. C. Grimsdale, A. B. Holmes, Angew. Chem. 1998, 110, 416;
10.1002/(SICI)1521-3757(19980216)110:4<416::AID-ANGE416>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1998, 37, 402;10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 2bA. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz, A. B Holmes, Chem. Rev. 2009, 109, 897.
- 3
- 3aT. Mizoroki, K. Mori, A. Ozaki, Bull. Chem. Soc. Jpn. 1971, 44, 581;
- 3bR. F. Heck, J. P. Nolley, Jr., J. Org. Chem. 1972, 37, 2320.
- 4J. E. Plevyak, R. F. Heck, J. Org. Chem. 1978, 43, 2454.
- 5For examples of differential Mizoroki–Heck reactions to construct unsymmetrical divinylbenzenes, see:
- 5aJ. E. Plevyak, J. E. Dickerson, R. F. Heck, J. Org. Chem. 1979, 44, 4078;
- 5bA. J. Spencer, J. Organomet. Chem. 1984, 265, 323;
- 5cW.-J. Tao, S. Nesbitt, R. F. Heck, J. Org. Chem. 1990, 55, 63;
- 5dA.-S. Carlström, T. Frejd, J. Org. Chem. 1991, 56, 1289;
- 5eS. Sengupta, S. K. Sadhukhan, Tetrahedron Lett. 1998, 39, 715;
- 5fC. Wang, L.-S. Tan, J.-P. He, H.-W. Hu, J.-H. Xu, Synth. Commun. 2003, 33, 773.
- 6For examples of differential cross-coupling reactions, see:
- 6aT. Oh-e, N. Miyaura, A. Suzuki, Synlett 1990, 221;
- 6bM. Rottländer, N. Palmer, P. Knochel, Synlett 1996, 573.
- 7For reviews, see:
- 7aC. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 2001, 34, 633;
- 7bL.-C. Campeau, D. R. Stuart, K. Fagnou, Aldrichimica Acta 2007, 40, 35;
- 7cX. Chen, K. M. Engle, D.-H Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 7dO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 7eT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147.
- 8For the initial report of PdII-mediated C(aryl)H olefination, see:
- 8aI. Moritani, Y. Fujiwara, Tetrahedron Lett. 1967, 8, 1119.
- 9For selected examples of subsequent work to establish catalytic turnover and expand the substrate scope, see:
- 9aY. Fujiwara, I. Moritani, S. Danno, R. Asano, S. Teranishi, J. Am. Chem. Soc. 1969, 91, 7166;
- 9bR. Shue, J. Chem. Soc. D 1971, 1510;
- 9cY. Fujiwara, R. Asano, I. Moritani, S. Teranishi, J. Org. Chem. 1976, 41, 1681;
- 9dJ. Tsuji, H. Nagashima, Tetrahedron 1984, 40, 2699;
- 9eC. Jia, W. Lu, T. Kitamura, Y. Fujiwara, Org. Lett. 1999, 1, 2097;
- 9fC. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura, Y. Fujiwara, Science 2000, 287, 1992.
- 10For examples of nondirected PdII-catalyzed CH olefination with nitrogen-containing heterocycles, see:
- 10aY. Fujiwara, O. Maruyama, M. Yoshidomi, H. Taniguchi, J. Org. Chem. 1981, 46, 851;
- 10bT. Itahara, M. Ikeda, T. Sakakibara, J. Chem. Soc. Perkin Trans. 1 1983, 1361;
- 10cE. M. Ferreira, B. M. Stoltz, J. Am. Chem. Soc. 2003, 125, 9578;
- 10dG. Abbiati, E. M. Beccalli, G. Broggini, C. Zoni, J. Org. Chem. 2003, 68, 7625;
- 10eS. Ma, S. Yu, Tetrahedron Lett. 2004, 45, 8419;
- 10fC. Liu, R. A. Widenhoefer, J. Am. Chem. Soc. 2004, 126, 10250;
- 10gN. P. Grimster, C. Gauntlett, C. R. A. Godfrey, M. J. Gaunt, Angew. Chem. 2005, 117, 3185;
10.1002/ange.200500468 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3125.
- 11For examples of PdII-catalyzed CH olefination using substrates containing directing groups, see:
- 11aM. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art, M. Nomura, J. Org. Chem. 1998, 63, 5211;
- 11bM. D. K. Boele, G. P. F. van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries, P. W. N. M. van Leeuwen, J. Am. Chem. Soc. 2002, 124, 1586;
- 11cV. G. Zaitsev, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 4156;
- 11dG. Cai, Y. Fu, Y. Li, X. Wan, Z. Shi, J. Am. Chem. Soc. 2007, 129, 7666;
- 11eC. E. Houlden, C. D. Bailey, J. G. Ford, M. R. Gagné, G. C. Lloyd-Jones, K. I. Booker-Milburn, J. Am. Chem. Soc. 2008, 130, 10066;
- 11fJ.-J. Li, T.-S. Mei, J.-Q. Yu, Angew. Chem. 2008, 120, 6552; Angew. Chem. Int. Ed. 2008, 47, 6452;
- 11gY. Lu, D.-H. Wang, K. M. Engle, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 5916.
- 12aE. Capito, J. M. Brown, A. Ricci, Chem. Commun. 2005, 1854;
- 12bA. García-Rubia, R. G. Arrayás, J. C. Carretero, Angew. Chem. 2009, 121, 6633;
10.1002/ange.200902802 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6511.
- 13S. H. Cho, S. J. Hwang, S. Chang, J. Am. Chem. Soc. 2008, 130, 9254.
- 14D.-H. Wang, K. M. Engle, B.-F. Shi, J.-Q. Yu, Science 2010, 327, 315.
- 15For an example of ligand-promoted PdII-catalyzed CH olefination with electron-deficient arenes, see: Y.-H. Zhang, B.-F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 5072.
- 16For examples of PdII-mediated CH olefination in total synthesis, see:
- 16aB. M. Trost, S. A. Godleski, J. P. Genêt, J. Am. Chem. Soc. 1978, 100, 3930;
- 16bT. D. Cushing, J. F. Sanz-Cervera, R. M. Williams, J. Am. Chem. Soc. 1993, 115, 9323;
- 16cP. S. Baran, E. J. Corey, J. Am. Chem. Soc. 2002, 124, 7904;
- 16dN. K. Garg, D. D. Caspi, B. M. Stoltz, J. Am. Chem. Soc. 2004, 126, 9552;
- 16eE. M. Beck, R. Hatley, M. J. Gaunt, Angew. Chem. 2008, 120, 3046; Angew. Chem. Int. Ed. 2008, 47, 3004;
- 16fA. L. Bowie, Jr., D. Trauner, J. Org. Chem. 2009, 74, 1581.
- 17For examples of arene–olefin coupling reactions using metals other than Pd, see:
- 17aL. N. Lewis, J. F. Smith, J. Am. Chem. Soc. 1986, 108, 2728;
- 17bS. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature 1993, 366, 529;
- 17cT. Matsubara, N. Koga, D. G. Musaev, K. Morokuma, J. Am. Chem. Soc. 1998, 120, 12692;
- 17dC. P. Lenges, M. Brookhart, J. Am. Chem. Soc. 1999, 121, 6616;
- 17eT. Matsumoto, D. J. Taube, R. A. Periana, H. Taube, H. Yoshida, J. Am. Chem. Soc. 2000, 122, 7414;
- 17fC.-H. Jun, J.-B. Hong, Y.-H. Kim, Y.-H. Chung, Angew. Chem. 2000, 112, 3582;
10.1002/1521-3757(20001002)112:19<3582::AID-ANGE3582>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3440;10.1002/1521-3773(20001002)39:19<3440::AID-ANIE3440>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 17gH. Weissman, X. Song, D. Milstein, J. Am. Chem. Soc. 2001, 123, 337;
- 17hR. K. Thalji, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 7192;
- 17iA. T. Luedtke, K. I. Goldberg, Angew. Chem. 2008, 120, 7808;
10.1002/ange.200800524 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7694.
- 18For a novel approach to construct 1,2,3-trisubstituted arenes through norbornene-mediated Pd0 catalysis, see:
- 18aM. Catellani, G. P. Chiusoli, J. Organomet. Chem. 1988, 346, C 27;
- 18bM. Catellani, F. Frignani, A. Rangoni, Angew. Chem. 1997, 109, 142;
10.1002/ange.19971090146 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 119. For a review, see:
- 18cD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174. For recent work, see:
- 18dB. Mariampillai, J. Alliot, M. Li, M. Lautens, J. Am. Chem. Soc. 2007, 129, 15372;
- 18eK. M. Gericke, D. I. Chai, N. Bieler, M. Lautens, Angew. Chem. 2009, 121, 1475; Angew. Chem. Int. Ed. 2009, 48, 1447.
- 19For rare examples of sequential CH activation of electron-rich heterocycles with PdII, see Refs. [10g, 12b, 16e] and the following:
- 19aM. Nakano, H. Tsurugi, T. Satoh, M. Miura, Org. Lett. 2008, 10, 1851;
- 19bS. Yanagisawa, K. Ueda, H. Sekizaawa, K. Itami, J. Am. Chem. Soc. 2009, 131, 14622.
- 20For a recent example of sequential ortho-CH olefination of 1-phenylpyrazoles using catalytic RhIII, see: N. Umeda, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2009, 74, 7094.
- 21For the use of mono-N-protected amino acid ligands in PdII-catalyzed enantioselective CH activation reactions, see:
- 21aB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. 2008, 120, 4960; Angew. Chem. Int. Ed. 2008, 47, 4882;
- 21bB.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 460.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.