Zinc(II) Containing γ-Keggin Sandwich-Type Silicotungstate: Synthesis in Organic Media and Oxidation Catalysis†
Yuji Kikukawa
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorKazuya Yamaguchi Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorNoritaka Mizuno Prof. Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorYuji Kikukawa
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorKazuya Yamaguchi Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorNoritaka Mizuno Prof. Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan), Fax: (+81) 3-5841-7220
Search for more papers by this authorWe thank Drs. K. Uehara and S. Uchida (The University of Tokyo) for their help with experiments. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Science, Sports and Technology of Japan. Y.K. is grateful for a JSPS Research Fellowship for Young Scientists.
Graphical Abstract
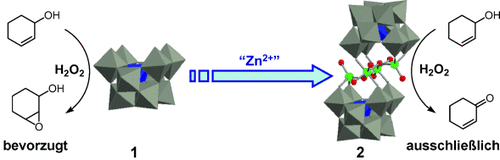
Zink macht den Unterschied: Das Polyoxometallat 1 reagiert mit Zn2+-Ionen in Aceton nahezu quantitativ zum neuartigen Sandwich-artigen POM 2. Die Oxidation sekundärer Alkohole mit H2O2 verläuft mit 2 effizient, und zwar mit Aktivitäten und Chemoselektivitäten, die sich von denen wolframhaltiger Katalysatoren einschließlich 1 erheblich unterscheiden (siehe Schema; grün Zn).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201001468_sm_miscellaneous_information.pdf304.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. L. Hill, C. M. Prosser-McCartha, Coord. Chem. Rev. 1995, 143, 407;
- 1bT. Okuhara, N. Mizuno, M. Misono, Adv. Catal. 1996, 41, 113;
- 1cR. Neumann, Prog. Inorg. Chem. 1998, 47, 317;
- 1dThematic issue on polyoxometalates. (Ed. C. L. Hill) Chem. Rev. 1998, 98, 1–390;
- 1eI. V. Kozhevnikov, Catalysis by Polyoxometalates, Wiley, Chichester, 2002.
- 2A. Tézé, G. Hervé, Inorg. Synth. 1990, 27, 85.
- 3
- 3aY. Nakagawa, K. Kamata, M. Kotani, K. Yamaguchi, N. Mizuno, Angew. Chem. 2005, 117, 5266;
10.1002/ange.200500491 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5136;
- 3bK. Kamata, S. Yamaguchi, M. Kotani, K. Yamaguchi, N. Mizuno, Angew. Chem. 2008, 120, 2441;
10.1002/ange.200705126 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2407;
- 3cY. Kikukawa, S. Yamaguchi, Y. Nakagawa, K. Uehara, S. Uchida, K. Yamaguchi, N. Mizuno, J. Am. Chem. Soc. 2008, 130, 15872.
- 4
- 4aA. Tézé, M. Michelon, G. Hervé, Inorg. Chem. 1997, 36, 5666;
- 4bB. Botar, P. Kögerler, C. L. Hill, Inorg. Chem. 2007, 46, 5398;
- 4cA. Sartorel, M. Carraro, G. Scorrano, R. De Zorzi, S. Geremia, N. D. McDaniel, S. Bernhard, M. Bonchio, J. Am. Chem. Soc. 2008, 130, 5006;
- 4dY. Kikukawa, S. Yamaguchi, K. Tsuchida, Y. Nakagawa, K. Uehara, K. Yamaguchi, N. Mizuno, J. Am. Chem. Soc. 2008, 130, 5472;
- 4eB. S. Bassil, S. S. Mal, M. H. Dickman, U. Kortz, H. Oelrich, L. Walder, J. Am. Chem. Soc. 2008, 130, 6696;
- 4fY. V. Geletii, B. Botar, P. Kögerler, D. A. Hillesheim, D. G. Musaev, C. L. Hill, Angew. Chem. 2008, 120, 3960;
10.1002/ange.200705652 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3896.
- 5
- 5aU. Kortz, Y. P. Jeannin, A. Tézé, G. Hervé, S. Isber, Inorg. Chem. 1999, 38, 3670;
- 5bU. Kortz, S. Isber, M. H. Dickman, D. Ravot, Inorg. Chem. 2000, 39, 2915;
- 5cF. Hussain, B. S. Bassil, L.-H. Bi, M. Reicke, U. Kortz, Angew. Chem. 2004, 116, 3567;
10.1002/ange.200454203 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3485;
- 5dL. Lisnard, P. Mialane, A. Dolbecq, J. Marrot, J. M. Clemente-Juan, E. Coronado, B. Keita, P. de Oliveira, L. Nadjo, F. Sécheresse, Chem. Eur. J. 2007, 13, 3525;
- 5eZ. Luo, P. Kögerler, R. Cao, I. Hakim, C. L. Hill, Dalton Trans. 2008, 54;
- 5fS. G. Mitchell, C. Ritchie, D.-L. Long, L. Cronin, Dalton Trans. 2008, 1415;
- 5gU. Kortz, S. Matta, Inorg. Chem. 2001, 40, 815;
- 5hZ. Zhang, Y. Li, E. Wang, X. Wang, C. Qin, H. An, Inorg. Chem. 2006, 45, 4313.
- 6
- 6aB. S. Bassil, M. H. Dickman, M. Reicke, U. Kortz, B. Keita, L. Nadjo, Dalton Trans. 2006, 4253;
- 6bH. Liu, J. Peng, Z. Su, Y. Chen, B. Dong, A. Tian, Z. Han, E. Wang, Eur. J. Inorg. Chem. 2006, 4827.
- 7It is well established that 3d-metal cations (especially M2+ ions) generally prefer to exist as monomeric species in aqueous media:
- 7aD. T. Richens, The Chemistry of Aqua Ions, Wiley, Chichester, 1997;
- 7bC. F. Base, Jr., R. E. Mesmer, The Hydrolysis of Cations, Wiley, New York, 1976.
- 8
- 8aK. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi, N. Mizuno, Science 2003, 300, 964;
- 8bK. Kamata, M. Kotani, K. Yamaguchi, S. Hikichi, N. Mizuno, Chem. Eur. J. 2007, 13, 639;
- 8cR. Ishimoto, K. Kamata, N. Mizuno, Angew. Chem. 2009, 121, 9062; Angew. Chem. Int. Ed. 2009, 48, 8900.
- 9It has been reported that the oxidation of 1 a with H2O2 by tungsten-based catalysts preferentially gives the epoxy alcohol 1 c rather than the enone 1 b:
- 9aD. Prat, B. Delpech, R. Lett, Tetrahedron Lett. 1986, 27, 711;
- 9bY. Ishii, K. Yamawaki, T. Ura, H. Yamada, T. Yoshida, M. Ogawa, J. Org. Chem. 1988, 53, 3587;
- 9cA. L. Villa de P., B. F. Sels, D. E. De Vos, P. A. Jacobs, J. Org. Chem. 1999, 64, 7267;
- 9dK. Sato, M. Aoki, M. Ogawa, T. Hashimoto, D. Panyella, R. Noyori, Bull. Chem. Soc. Jpn. 1997, 70, 905.
- 10It would be very advantageous for practical applications if the reactions could be carried out with in-situ-prepared catalysts, by simply mixing the required components (even without isolation of truly active catalysts). Although the in-situ-prepared POM-based catalysts in aqueous media with the pH value controlled (actually using synthetic solutions as catalysts) have been reported to be active for aerobic lignin oxidation[10a] and the oxidation of alcohols with H2O2,[10b] similar in-situ-prepared catalysts in organic media have not been reported to our knowledge:
- 10aI. A. Weinstock, E. M. G. Barbuzzi, M. W. Wemple, J. J. Cowan, R. S. Reiner, D. M. Sonnen, R. A. Heintz, J. S. Bond, C. L. Hill, Nature 2001, 414, 191;
- 10bD. Sloboda-Rozner, P. L. Alster, R. Neumann, J. Am. Chem. Soc. 2003, 125, 5280.
- 11Transition-metal-substituted POMs have usually been synthesized by the reaction of alkali-metal salts of lacunary POMs and the corresponding transition-metal salts in aqueous media. To date, there are only a few reports on the synthesis in organic media. The strategy to use organic media and to control the reactivities of POMs with counter cations demonstrated herein will open up a new avenue for POM chemistry:
- 11aA. M. Khenkin, L. J. W. Shimon, R. Neumann, Eur. J. Inorg. Chem. 2001, 789;
- 11bK. Yamaguchi, N. Mizuno, New J. Chem. 2002, 26, 972;
- 11cP. Mialane, C. Duboc, J. Marrot, E. Rivière, A. Dolbecq, F. Sécheresse, Chem. Eur. J. 2006, 12, 1950.
- 12Very recently, Mialane and co-workers reported the synthesis of a sandwich-type POM Cs10[(γ-SiW10O36)2{Cr(OH)(OH2)}3]⋅17 H2O by the reaction of [γ-SiW10O36]8− with the trinuclear [Cr3(OAc)7(OH)2] complex in aqueous medium. They mentioned that the choice of the chromium precursor was crucial for the synthesis: J.-D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekallé, J. Marrot, F. Sécheresse, C. Duboc, E. Rivière, Inorg. Chem. 2010, 49, 2851.
- 13CCDC-768702 (TBA-Zn4) contains the supplementary crystallographic data for this paper. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. It was confirmed by IR measurements that TBA-Zn4 was thermally stable upon the heat treatment at least up to 80 °C.
- 14aT. J. R. Weakley, J. Chem. Soc. Dalton Trans. 1973, 341;
- 14bJ. Wang, L. Yan, G. Li, X. Wang, Y. Ding, J. Suo, Tetrahedron Lett. 2005, 46, 7023;
- 14cM. Piepenbrink, E. M. Limanski, B. Krebs, Z. Anorg. Allg. Chem. 2002, 628, 1187;
- 14dC. M. Tourné, G. F. Tourné, F. J. Zonnevijlle, J. Chem. Soc. Dalton Trans. 1991, 143;
- 14eR. Neumann, A. M. Khenkin, Inorg. Chem. 1995, 34, 5753;
- 14fR. Neumann, A. M. Khenkin, J. Mol. Catal. 1996, 114, 169;
- 14gU. Kortz, N. K. Al-Kassem, M. G. Savelieff, N. A. Al Kadi, M. Sadakane, Inorg. Chem. 2001, 40, 4742;
- 14hD. Drewes, E. M. Limanski, B. Krebs, Eur. J. Inorg. Chem. 2005, 1542;
- 14iH. Tan, Z. Zhang, D. Liu, Y. Qi, E. Wang, Y. Li, J. Cluster Sci. 2008, 19, 543;
- 14jM. Kozik, L. C. W. Baker, J. Am. Chem. Soc. 1987, 109, 3159;
- 14kS.-T. Zheng, J. Zhang, G.-Y. Yang, Eur. J. Inorg. Chem. 2004, 2004.
- 15aJ. Tao, M.-L. Tong, J.-X. Shi, X.-M. Chen, S. W. Ng, Chem. Commun. 2000, 2043;
- 15bM. Alexiou, E. Katsoulakou, C. Dendrinou-Samara, C. P. Raptopoulou, V. Psycharis, E. Manessi-Zoupa, S. P. Perlepes, D. P. Kessissoglou, Eur. J. Inorg. Chem. 2005, 1964.
- 16Owing to increasing environmental concerns, many efforts have been made to develop alcohol oxidation systems using environmentally benign H2O2 or O2 (air) as a sole oxidant:
- 16aR. A. Sheldon, I. W. C. E. Arends, D. Dijisman, Catal. Today 2000, 57, 157;
- 16bT. Mallat, A. Baiker, Chem. Rev. 2004, 104, 3037;
- 16cT. Matsumoto, M. Ueno, N. Wang, S. Kobayashi, Chem. Asian J. 2008, 3, 196.
- 17The aerobic alcohol oxidations are generally carried out at relatively high reaction temperatures.[16] Fine chemicals have various functional groups and their thermal stabilities are generally low. Thus, the oxidations should often be carried out under mild reaction conditions with high functional-group tolerance. In addition, the simplicity of operation is also very important from an economic point of view. For these reasons, H2O2 could be the oxidant of choice for alcohol oxidations (especially for fine chemicals): R. A. Sheldon, J. Chem. Technol. Biotechnol. 1997, 68, 381, and see also ref. [16a].
- 18The TBA-Zn4-catalyzed oxidation of benzyl alcohol under the conditions described in Table 2 gave benzaldehyde and benzoic acid in 87 % and 3 % yields (for 2.5 h), respectively.
- 19Acetone was not a special solvent; for example, the oxidation of 1 a in acetonitrile gave 1 b in>99 % yield under the same conditions.
- 20Even with an equimolar amount of H2O2 with respect to alcohols, the oxidation efficiently proceeded; for example, the TBA-Zn4-catalyzed oxidations of 1 a, 3 a, and 8 a (0.5 mmol) using 1 equivalent of H2O2 with respect to alcohols (other conditions were the same as those described in Table 2) gave the corresponding ketones in 99 % (for 2 h), 94 % (for 3 h), and 93 % (for 4.5 h) yields, respectively.
- 21It has been reported that the H2O2-based oxidations with metal-substituted POMs (Al3+ or Zn2+) show high chemoselectivity to alcoholic functions:
- 21aJ. Wang, L. Yan, G. Qian, S. Li, K. Yang, H. Liu, X. Wang, Tetrahedron 2007, 63, 1832;
- 21bJ. Wang, L. Yan, G. Li, X. Wang, Y. Ding, J. Suo, Tetrahedron Lett. 2007, 46, 7023;
- 21cG. Maayan, R. H. Fish, R. Neumann, Org. Lett. 2003, 5, 3547.
- 22α,β-Alkynic ketones are very important compounds and have been utilized as precursors for heterocyclic compounds and DNA-cleavage reagents. Although several stoichiometric oxidants such as MnO2, Swern reagent, and Dess–Martin reagent have been utilized for the oxidation of propargylic alcohols, the catalytic procedures with H2O2 or O2 are quite limited.[16]
- 23The oxidation of 1 a (under the conditions described in Table 1) did not proceed at all in the presence of a mixture of 1) Na2WO4⋅2 H2O (8 mol %) and TBABr (6.4 mol %), or 2) Na2WO4⋅2 H2O (8 mol %), [Zn(acac)2] (1.6 mol %), and TBABr (6.4 mol %). These results can rule out any contribution to the observed catalysis from tungsten- and/or zinc-based impurities, and the observed catalysis is intrinsically derived from TBA-Zn4.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.