A Modular Synthetic Approach to Conjugated Pentacene Di-, Tri-, and Tetramers†
Dan Lehnherr
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Search for more papers by this authorAdrian H. Murray
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Search for more papers by this authorRobert McDonald Dr.
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorRik R. Tykwinski Prof. Dr.
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Present address: Institut für Organische Chemie, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Henkestrasse 42, 91054 Erlangen (Germany)
Search for more papers by this authorDan Lehnherr
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Search for more papers by this authorAdrian H. Murray
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Search for more papers by this authorRobert McDonald Dr.
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorRik R. Tykwinski Prof. Dr.
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 (Canada), Fax: (+1) 780-492-8231
Present address: Institut für Organische Chemie, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Henkestrasse 42, 91054 Erlangen (Germany)
Search for more papers by this authorThis work was generously supported by the University of Alberta, the Natural Sciences and Engineering Research Council of Canada (NSERC) through the Discovery grant program, and the Canadian Foundation for Innovation (CFI). D.L. thanks NSERC (PGS-D), the Alberta Ingenuity Fund (AIF) , the Alberta Heritage Fund, the Killam Trusts, and the University of Alberta for scholarship support. A.H.M. thanks NSERC (PGS-D) and AIF for scholarship support.
Graphical Abstract
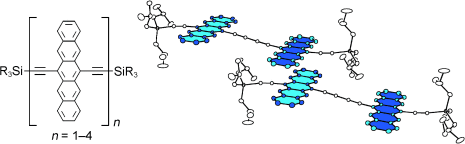
Achtung, Bandlücke! Di-, tri- und tetramere Pentacene können aus einem vielfältig einsetzbaren Baustein durch Hay-Homokupplungen und auch durch Cadiot-Chodkiewicz-Kreuzkupplungen synthetisiert werden. Durch diese modulare Herangehensweise können Löslichkeit, Stabilität und HOMO-LUMO-Bandlücke der Verbindungen in Abhängigkeit von ihrer Länge bewertet werden. Im iBu3Si-substituierten Pentacen-Dimer treten weit reichende dreidimensionale π-Überlappungen auf (siehe Bild; n=2).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201000555_sm_miscellaneous_information.pdf6.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. E. Anthony, Chem. Rev. 2006, 106, 5028–5048;
- 1bJ. E. Anthony, Angew. Chem. 2008, 120, 460–492;
10.1002/ange.200604045 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 452–483.
- 2
- 2aS. Tokito, K.-H. Weinfurtner, H. Fujikawa, T. Tsutsui, Y. Taga, Proc. SPIE-Int. Soc. Opt. Eng. 2001, 4105, 69–74;
- 2bT. Okamoto, Z. Bao, J. Am. Chem. Soc. 2007, 129, 10308–10309;
- 2cT. Okamoto, Y. Jiang, F. Qu, A. C. Mayer, J. E. Parmer, M. D. McGehee, Z. Bao, Macromolecules 2008, 41, 6977–6980.
- 3
- 3aD. Lehnherr, R. R. Tykwinski, Materials 2010, 3, 2772–2800;
- 3bD. Lehnherr, R. R. Tykwinski, Org. Lett. 2007, 9, 4583–4586;
- 3cD. Lehnherr, R. McDonald, M. J. Ferguson, R. R. Tykwinski, Tetrahedron 2008, 64, 11449–11461;
- 3dD. Lehnherr, J. Gao, F. A. Hegmann, R. R. Tykwinski, Org. Lett. 2008, 10, 4779–4782;
- 3eD. Lehnherr, J. Gao, F. A. Hegmann, R. R. Tykwinski, J. Org. Chem. 2009, 74, 5017–5024;
- 3fX. Zhang, X. Jiang, J. Luo, C. Chi, H. Chen, J. Wu, Chem. Eur. J. 2010, 16, 464–468.
- 4
- 4aH. Meier, Angew. Chem. 2005, 117, 2536–2561;
10.1002/ange.200461146 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2482–2506;
- 4bR. E. Martin, F. Diederich, Angew. Chem. 1999, 111, 1440–1469;
10.1002/(SICI)1521-3757(19990517)111:10<1440::AID-ANGE1440>3.0.CO;2-H Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1350–1377;10.1002/(SICI)1521-3773(19990517)38:10<1350::AID-ANIE1350>3.0.CO;2-6 PubMed Web of Science® Google Scholar
- 4c Electronic Materials—The Oligomer Approach (Eds.: ), Wiley-VCH, Weinheim, 1998.
- 5For representative synthetic methods to the pentacene framework, see:
- 5aM. M. Payne, S. A. Odom, S. R. Parkin, J. E. Anthony, Org. Lett. 2004, 6, 3325–3328;
- 5bT. Takahashi, M. Kitamura, B. Shen, K. Nakjima, J. Am. Chem. Soc. 2000, 122, 12876–12877;
- 5cJ. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml, S. Bernhard, N. Byrne, L. R. Kim, R. A. Pascal, Jr., J. Am. Chem. Soc. 2006, 128, 17043–17050;
- 5dQ. Miao, X. Chi, S. Xiao, R. Zeis, M. Lefenfeld, T. Siegrist, M. L. Steigerwald, C. Nuckolls, J. Am. Chem. Soc. 2006, 128, 1340–1345;
- 5eJ. E. Rainbolt, G. P. Miller, J. Org. Chem. 2007, 72, 3020–3030;
- 5fY. Zhao, R. Mondal, D. C. Neckers, J. Org. Chem. 2008, 73, 5506–5513.
- 6
- 6a Carbon-Rich Compounds (Eds.: ), Wiley-VCH, Weinheim, 2006;
- 6b Acetylene Chemistry: Chemistry, Biology, and Material Science (Eds.: ), Wiley-VCH, Weinheim, 2005;
- 6cP. Siemsen, R. C. Livingston, F. Diederich, Angew. Chem. 2000, 112, 2740–2767;
10.1002/1521-3757(20000804)112:15<2740::AID-ANGE2740>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2632–2657.10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 7
- 7aD. Lehnherr, R. McDonald, R. R. Tykwinski, Org. Lett. 2008, 10, 4163–4166;
- 7bD. Lehnherr, A. H. Murray, R. McDonald, M. J. Ferguson, R. R. Tykwinski, Chem. Eur. J. 2009, 15, 12580–12584.
- 8In situ generation and derivatization of precursor 2 has been, to the best of our knowledge, successful in two cases, see:
- 8aK. Susumu, T. V. Duncan, M. J. Therien, J. Am. Chem. Soc. 2005, 127, 5186–5195;
- 8bC.-Y. Lin, Y.-C. Wang, S.-J. Hsu, C.-F. Lo, E. W.-G. Diau, J. Phys. Chem. C 2010, 114, 687–693.
- 9For the elegant use of a similar protection method for the formation of anthracene polymers, see: M. S. Taylor, T. M. Swager, Angew. Chem. 2007, 119, 8632–8635; Angew. Chem. Int. Ed. 2007, 46, 8480–8483.
- 10H. Hofmeister, K. Annen, H. Laurent, R. Wiechert, Angew. Chem. 1984, 96, 720–722; Angew. Chem. Int. Ed. Engl. 1984, 23, 727–729.
- 11See the Supporting Information for X-ray crystallographic structures and details. CCDC- 760494 (12 a), 760495 (12 b), 760496 (13 a), 760497 (13 b), 760498 (13 d), 760499 (14 e), 760500 (15 e) contain the supplementary crystallographic data for these compounds. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12A. S. Hay, J. Org. Chem. 1962, 27, 3320–3321.
- 13Unprotected analogues to 13 a that contain two hydroxy moieties were found to be unsuitable for metal-mediated coupling reactions such as those presented herein, see reference [7a].
- 14W. Chodkiewicz, Ann. Chim. (Paris) 1957, 2, 819–869.
- 15Desilylation of 15 d with CsF in 5:1 THF/H2O, or KF in 5:1 THF/H2O resulted in recovery of the starting material.
- 16Monomers 5 a–c were synthesized by addition of the corresponding lithium acetylide to 6,13-pentacenequinone followed by treatment of the resulting diol with SnCl2⋅2H2O. See the Supporting Information for details.
- 17The value used as the absorption edge corresponds to the lowest-energy absorption wavelength with a molar absorptivity (ε) ≥1000 L mol−1 cm−1.
- 18
- 18aS. S. Zade, M. Bendikov, Angew. Chem. 2010, 122, 4104–4107;
10.1002/ange.200906002 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4012–4015;
- 18bM. M. Payne, S. R. Parkin, J. E. Anthony, J. Am. Chem. Soc. 2005, 127, 8028–8029;
- 18cD. Chun, Y. Cheng, F. Wudl, Angew. Chem. 2008, 120, 8508–8513;
10.1002/ange.200803345 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8380–8385.
- 19Fluorescence quantum efficiencies were obtained relative to cresyl violet perchlorate in methanol, see: S. J. Isak, E. M. Eyring, J. Phys. Chem. 1992, 96, 1738–1742.
- 20See the Supporting Information for cyclic voltammograms and a complete table of redox potentials in CH2Cl2 and THF.
- 21X-ray data for 6 b⋅2 CHCl3: C78H80Cl6Si2, Mr=1286.30; crystal dimensions (mm) 0.54×0.44×0.08; triclinic space group
 (No. 2); a=10.1229(10), b=11.4926(11), c=15.3308(15) Å; α=101.5627(12), β=91.9451(12), γ=96.5342(12)°; V=1733.1(3) Å3; Z=1; ρcalcd=1.232 g cm−3; μ=0.325 mm−1; λ=0.71073 Å; T=−100 °C; 2θmax=51.52°; total data collected=12 984; R1=0.0695 [5119 observed reflections with Fo2≥2σ(Fo2)]; wR2=0.2102 for 439 variables, 13 restraints, and 6603 unique reflections; residual electron density=0.720 and −0.552 e Å−3. The C(34A)–C(35A) and C(34B)–C(35B) distances (within a partially disordered silyl isobutyl group) were constrained to be equal (within 0.01 Å) during refinement. The Cl–C distances (Cl(1S)–C(1S), Cl(2S)–C(1S), Cl(1S)–C(3S), Cl(4S)–C(2S), Cl(5S)–C(2S), Cl(6S)–C(2S) within the disordered solvent chloroform molecule were constrained to be equal (within 0.03 Å) to a common value, as were the Cl–Cl distances [Cl(1S)–Cl(2S), Cl(1S)–Cl(3S), Cl(2S)–Cl(3S), Cl(4S)–Cl(5S), Cl(4S)–Cl(6S), Cl(5S)–Cl(6S). CCDC- 760493 contains the supplementary crystallographic data for this compound. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.)
(No. 2); a=10.1229(10), b=11.4926(11), c=15.3308(15) Å; α=101.5627(12), β=91.9451(12), γ=96.5342(12)°; V=1733.1(3) Å3; Z=1; ρcalcd=1.232 g cm−3; μ=0.325 mm−1; λ=0.71073 Å; T=−100 °C; 2θmax=51.52°; total data collected=12 984; R1=0.0695 [5119 observed reflections with Fo2≥2σ(Fo2)]; wR2=0.2102 for 439 variables, 13 restraints, and 6603 unique reflections; residual electron density=0.720 and −0.552 e Å−3. The C(34A)–C(35A) and C(34B)–C(35B) distances (within a partially disordered silyl isobutyl group) were constrained to be equal (within 0.01 Å) during refinement. The Cl–C distances (Cl(1S)–C(1S), Cl(2S)–C(1S), Cl(1S)–C(3S), Cl(4S)–C(2S), Cl(5S)–C(2S), Cl(6S)–C(2S) within the disordered solvent chloroform molecule were constrained to be equal (within 0.03 Å) to a common value, as were the Cl–Cl distances [Cl(1S)–Cl(2S), Cl(1S)–Cl(3S), Cl(2S)–Cl(3S), Cl(4S)–Cl(5S), Cl(4S)–Cl(6S), Cl(5S)–Cl(6S). CCDC- 760493 contains the supplementary crystallographic data for this compound. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.)
- 22Interplanar distances were calculated from the distance between the least-squares plane generated from the acene carbon atoms of one pentacene moiety and that of its noncovalent neighboring moiety.
- 23For a related crystal structure of a conjugated anthracene–pentacene dyad, see reference [7b].
- 24H. Pang, F. Vilela, P. J. Skabara, J. J. W. McDouall, D. J. Crouch, T. D. Anthopoulos, D. D. C. Bradley, D. M. de Leeuw, P. N. Horton, M. B. Hursthouse, Adv. Mater. 2007, 19, 4438–4442.
- 25While red-shifted λmax values for 6 b are qualitatively consistent with π stacking observed in the crystallographic analysis, more definitive conclusions are tenuous without additional data regarding the morphology of crystals of 6 b grown from THF or knowledge of the film morphology used for the UV/Vis analyses. Crystals of 6 b were grown from THF/MeOH and CH2Cl2 solutions, but unfortunately, neither data set was suitable for publication.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.