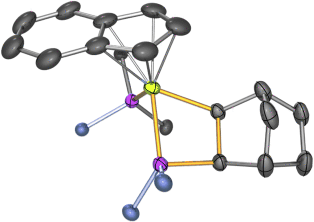
Concerted [2+2] Cycloaddition of Alkenes to a Ruthenium–Phosphorus Double Bond†
Eric J. Derrah
Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, BC V8W3V6 (Canada), Fax: (+1) 250-721-7147 http://web.uvic.ca/∼lisarose/
Search for more papers by this authorDimitrios A. Pantazis Dr.
Institute for Physical and Theoretical Chemistry, University of Bonn, 53115 Bonn (Germany)
Search for more papers by this authorRobert McDonald Dr.
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, AB T6G 2G2 (Canada)
Search for more papers by this authorLisa Rosenberg Prof.
Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, BC V8W3V6 (Canada), Fax: (+1) 250-721-7147 http://web.uvic.ca/∼lisarose/
Search for more papers by this authorEric J. Derrah
Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, BC V8W3V6 (Canada), Fax: (+1) 250-721-7147 http://web.uvic.ca/∼lisarose/
Search for more papers by this authorDimitrios A. Pantazis Dr.
Institute for Physical and Theoretical Chemistry, University of Bonn, 53115 Bonn (Germany)
Search for more papers by this authorRobert McDonald Dr.
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, AB T6G 2G2 (Canada)
Search for more papers by this authorLisa Rosenberg Prof.
Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, BC V8W3V6 (Canada), Fax: (+1) 250-721-7147 http://web.uvic.ca/∼lisarose/
Search for more papers by this authorWe are grateful to the NSERC of Canada for financial support.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201000356_sm_miscellaneous_information.pdf948.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. J. Derrah, K. E. Giesbrecht, R. McDonald, L. Rosenberg, Organometallics 2008, 27, 5025;
- 1bE. J. Derrah, D. A. Pantazis, R. McDonald, L. Rosenberg, Organometallics 2007, 26, 1473.
- 2D. S. Glueck, Chem. Eur. J. 2008, 14, 7108.
- 3For examples see the following, and references therein;
- 3aJ. F. Hartwig, Nature 2008, 455, 314;
- 3bT. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795.
- 4
- 4aP. Butti, R. Rochat, A. D. Sadow, A. Togni, Angew. Chem. 2008, 120, 4956;
10.1002/ange.200801287 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4878;
- 4bM. O. Shulyupin, I. G. Trostyanskaya, V. A. Kazankova, I. P. Beletskaya, Russ. J. Org. Chem. 2006, 42, 17;
- 4cA. D. Sadow, A. Togni, J. Am. Chem. Soc. 2005, 127, 17012;
- 4dF. Jérôme, F. Monnier, H. Lawicka, S. Derien, P. H. Dixneuf, Chem. Commun. 2003, 696.
- 5
- 5aV. S. Chan, M. Chiu, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6021;
- 5bD. S. Glueck, Synlett 2007, 2627;
- 5cC. Scriban, D. S. Glueck, L. N. Zakharov, W. S. Kassel, A. G. DiPasquale, J. A. Golen, A. L. Rheingold, Organometallics 2006, 25, 5757;
- 5dM. O. Shulyupin, M. A. Kazankova, I. P. Beletskaya, Org. Lett. 2002, 4, 761;
- 5eM. A. Kazankova, M. O. Shulyupin, I. P. Beletskaya, Synlett 2003, 2155. In reference [5 d] the authors proposed a mechanism which includes alkene insertion into a MH bond, but they later revise this interpretation (Ref. [5 e]) and describe the mechanism as a Michael-type addition onto activated aryl alkenes, noting that these reactions do not proceed for simple alkenes.
- 6K. Takaki, K. Komeyama, D. Kobayashi, T. Kawabata, K. Takehira, J. Alloys Compd. 2006, 408, 432.
- 7aA. M. Kawaoka, M. R. Douglass, T. J. Marks, Organometallics 2003, 22, 4630;
- 7bA. Motta, I. L. Fragala, T. J. Marks, Organometallics 2005, 24, 4995.
- 8M. R. Crimmin, A. G. M. Barrett, M. S. Hill, P. B. Hitchcock, P. A. Procopiou, Organometallics 2007, 26, 2953.
- 9D. K. Wicht, D. S. Glueck in Catalytic Heterofunctionalization. From Hydroamination to Hydrozirconation (Ed.: ), Wiley-VCH, Weinheim, 2001, p. 143.
10.1002/3527600159.ch5 Google Scholar
- 10See the Supporting Information for details. Similar data obtained for 2 a was complicated by overlap of the peaks of interest with those from the Cy groups.
- 11Crystallographic data for 2 a: C42H47NP2Ru, Mr=728.82; crystal dimensions (mm)0.57×0.28×0.28; monocclinic space group P21/n (an alternate setting of P21/c [No. 14]); a=13.1935(8), b=16.9872(11), c=15.6856(10) Å; β=91.1278(9)°; V=3514.8(4) Å3; Z=4; ρcalcd=1.377 g cm−3; μ=0.568 mm−1; λ=0.71073 Å; T=−80 °C; 2θmax=52.80°; total data collected=27 646; R1=0.0229 (6571 observed reflections with Fo2≥2σ(Fo2)); wR2=0.0632 for 415 variables and all 7203 unique reflections; residual electron density=0.532 and −0.247 e Å−3.
- 12F. Schaper, S. R. Foley, R. F. Jordan, J. Am. Chem. Soc. 2004, 126, 2114.
- 13See for example Ref. [5c] and
- 13aW. Malisch, B. Klupfel, D. Schumacher, M. Nieger, J. Organomet. Chem. 2002, 661, 95.
- 14We were limited in our choice of polar solvent because of the reactivity of 1 a,b with nitriles as well as halide-containing or protic solvents (see Ref. [1a]).
- 15Solvent effects on rate are particularly pronounced for zwitterionic intermediates in [2+2] additions, which are generally less successful in achieving conformations that minimize the coulombic separation than are those in, for example, [2+4] cycloadditions;
- 15aR. Huisgen, Acc. Chem. Res. 1977, 10, 117;
- 15bR. Huisgen, Pure Appl. Chem. 1980, 52, 2283.
- 16Examples of the utility of [D2]ethylene in diagnosing mechanisms of cycloaddition reactions include:
- 16aP. D. Bartlett, R. A. Minns, K. Hummel, S. P. Elliott, C. M. Sharts, G. M. Cohen, J. Y. Fukunaga, J. Am. Chem. Soc. 1972, 94, 2898;
- 16bK. N. Houk, Y. T. Lin, F. K. Brown, J. Am. Chem. Soc. 1986, 108, 554;
- 16cH. B. Liu, R. J. Hamers, J. Am. Chem. Soc. 1997, 119, 7593;
- 16dP. P. Nicholas, R. T. Carroll, J. Org. Chem. 1968, 33, 2345.
- 17We also examined the addition of cis- and trans-2-butene to 1 a,b, but these internal alkenes reacted very slowly, allowing competing orthometalation of 1 a,b (see Ref. [1b]). The somewhat ambiguous results of these experiments (see the Supporting Information) point to a stereochemical preference, in this sterically congested system, for the formation of cisoid metallacycles, which is avoided through the use of cis- and trans-[D2]ethylene.
- 18In the 1H NMR spectra of cis- and trans-[D2]-3 a,b, peaks resulting from the metallacycle protons show reduced multiplicities and intensities relative to those in the spectra of [D0]-3 a,b. 2H NMR spectra confirmed the equal distribution of deuterium throughout all four positions on the metallacycle.
- 19Crystal data for 5 a: C46H54P2Ru, Mr=769.90; crystal dimensions (mm)0.54×0.40×0.22; monocclinic space group P21/n (an alternate setting of P21/c [No. 14]); a=11.6081(7), b=17.4813(11), c=19.5737(12) Å; β=105.6999(8)°; V=3823.8(4) Å3; Z=4; ρcalcd=1.337 g cm−3; μ=0.525 mm−1; λ=0.71073 Å; T=−80 °C; 2θmax=54.98°; total data collected=33 061; R1=0.0272 (7822 observed reflections with Fo2≥2σ(Fo2)); wR2=0.0728 for 442 variables and all 8721 unique reflections; residual electron density=0.622 and −0.264 e Å−3.
- 20Addition of excess ethylvinylether, an electron-rich terminal alkene of intermediate bulk, to 1 a,b gave quantitative [2+2] cycloaddition within 30 min at RT (a rate intermediate between acrylonitrile and 1-hexene), but gave the syn and anti isomers (7 a,b) in a 1:1 ratio. It is not yet clear whether this selectivity arises from transition state effects in the original cycloaddition (i.e. a purely kinetic phenomenon) or whether the electron-rich nature of the resulting metallacycle hastens equilibration of these isomers (DFT calculations again indicate that these syn and anti isomers are isoenergetic).
- 21We have seen no evidence for the reversibility of these [2+2] cycloaddition reactions (i.e., no free alkene appears in solution samples of the purified metallacyclic products). 31P{1H} NMR analysis of sealed samples of 2 a,b showed a slight increase in the relative amount of the anti isomers (from <5 % to ca. 10 %) over a year at RT, consistent with extremely slow equilibration, presumably via reversible dissociation of the phosphine end of the metallacycle, which would allow the requisite bond rotation(s) and inversion at Ru to give epimerization at the α-carbon atom.
- 22J. W. Tye, J. F. Hartwig, J. Am. Chem. Soc. 2009, 131, 14703.
- 23Other relevant cycloadditions at MP double bonds include the following. [2+1] cycloaddition of alkenes and alkynes at metal phosphinidenes (M=PR):
- 23aM. L. G. Borst, N. van der Riet, R. H. Lemmens, F. J. J. de Kanter, M. Schakel, A. W. Ehlers, A. M. Mills, M. Lutz, A. L. Spek, K. Lammertsma, Chem. Eur. J. 2005, 11, 3631;
- 23bM. L. G. Borst, R. E. Bulo, D. J. Gibney, Y. Alem, F. J. J. de Kanter, A. W. Ehlers, M. Schakel, M. Lutz, A. L. Spek, K. Lammertsma, J. Am. Chem. Soc. 2005, 127, 16985;
- 23cA. Marinetti, F. Mathey, Organometallics 1984, 3, 456. This last study gives evidence pointing to a concerted mechanism for this route to metal-bound phosphiranes. [2+2] cycloaddition of alkynes with a zirconium phosphinidene complex to give metallacyclobutene complexes:
- 23dT. L. Breen, D. W. Stephan, Organometallics 1996, 15, 5729. Stoichiometric [2+2] cycloaddition of isocyanates and isothiocyanates with group 6 phosphenium complexes (M=P(δ+)R2):
- 23eW. Malisch, K. Grun, O. Fey, C. A. El Baky, J. Organomet. Chem. 2000, 595, 285;
- 23fW. Malisch, C. Hahner, K. Grun, J. Reising, R. Goddard, C. Kruger, Inorg. Chim. Acta 1996, 244, 147;
- 23gH. Pfister, W. Malisch, J. Organomet. Chem. 1992, 439, C 11. The mechanism of these cycloadditions (Refs. [23 e–g]) has not been studied.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.