Macrocyclization by Nickel-Catalyzed, Ester-Promoted, Epoxide–Alkyne Reductive Coupling: Total Synthesis of (−)-Gloeosporone†
James D. Trenkle Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 http://web.mit.edu/chemistry/jamison
Current address: Gilead Sciences, 333 Lakeside Dr., Foster City, CA 94404 (USA)
Search for more papers by this authorTimothy F. Jamison Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 http://web.mit.edu/chemistry/jamison
Search for more papers by this authorJames D. Trenkle Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 http://web.mit.edu/chemistry/jamison
Current address: Gilead Sciences, 333 Lakeside Dr., Foster City, CA 94404 (USA)
Search for more papers by this authorTimothy F. Jamison Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 http://web.mit.edu/chemistry/jamison
Search for more papers by this authorSupport for this work was provided by the National Institute of General Medical Sciences (GM-72566). We are grateful to Li Li for obtaining mass spectrometric data for all compounds (MIT Department of Chemistry Instrumentation Facility, which is supported in part by the NSF (CHE-9809061 and DBI-9729592) and the NIH (1S10RR13886-01)).
Abstract
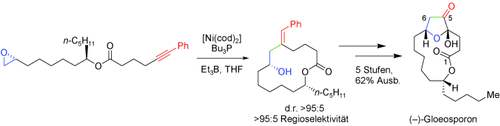
Im Zentrum der Totalsynthese der Titelverbindung steht eine neuartige Strategie, bei der mithilfe von Triethylboran und einem Nickel(0)-Phosphan-Komplex effizient ein 14-gliedriger Ring geschlossen wird (siehe Schema; cod=Cyclooctadien). Die Synthese verläuft über zehn Stufen in ca. 9 % Gesamtausbeute.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200902079_sm_miscellaneous_information.pdf5.4 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Parenty, X. Moreau, J.-M. Campagne, Chem. Rev. 2006, 106, 911.
- 2Ring-closing metathesis:
- 2aA. Gradillas, J. Perez-Castells, Angew. Chem. 2006, 118, 6232;
10.1002/ange.200600641 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6086; CH oxidation:
- 2bK. J. Fraunhoffer, N. Prabagaran, L. E. Sirois, M. C. White, J. Am. Chem. Soc. 2006, 128, 9032; nickel-mediated reactions:
- 2cK. Namba, Y. Kishi, J. Am. Chem. Soc. 2005, 127, 15382.
- 3
- 3aC. Molinaro, T. F. Jamison, J. Am. Chem. Soc. 2003, 125, 8076;
- 3bK. S. Woodin, T. F. Jamison, J. Org. Chem. 2007, 72, 7451.
- 4
- 4aA. R. Lax, G. E. Templeton, W. L. Meyer, Phytopathology 1985, 75, 386;
- 4bW. L. Meyer, A. R. Lax, G. E. Templeton, M. J. Brannon, Tetrahedron Lett. 1983, 24, 5059;
- 4cR. W. Carling, A. B. Holmes, Tetrahedron Lett. 1986, 27, 6133;
- 4dW. L. Meyer, D. Seebach, S. L. Schreiber, W. B. Schweizer, A. K. Beck, W. Scheifele, S. E. Kelly, Helv. Chim. Acta 1987, 70, 281.
- 5aG. Adam, R. Zibuck, D. Seebach, J. Am. Chem. Soc. 1987, 109, 6176;
- 5bS. L. Schreiber, S. E. Kelly, J. A. Porco, T. Sammakia, E. M. Suh, J. Am. Chem. Soc. 1988, 110, 6210;
- 5cS. Takano, Y. Shimazaki, M. Takahashi, K. Ogasawara, J. Chem. Soc. Chem. Commun. 1988, 1004;
- 5dD. Seebach, G. Adam, R. Zibuck, W. Simon, M. Rouilly, W. L. Meyer, J. F. Hinton, T. A. Privett, G. E. Templeton, D. K. Heiny, U. Gisi, H. Binder, Liebigs Ann. Chem. 1989, 1233;
- 5eN. R. Curtis, A. B. Holmes, M. G. Looney, N. D. Pearson, G. C. Slim, Tetrahedron Lett. 1991, 32, 537;
- 5fM. Matsushita, M. Yoshida, Y. Zhang, M. Miyashita, H. Irie, T. Ueno, T. Tsurushima, Chem. Pharm. Bull. 1992, 40, 524;
- 5gA. Sharma, S. Gamre, S. Chattopadhyay, Lett. Org. Chem. 2005, 2, 547;
- 5hA. Fürstner, K. Langemann, J. Am. Chem. Soc. 1997, 119, 9130;
- 5iS. V. Ley, E. Cleator, J. Harter, C. J. Hollowood, Org. Biomol. Chem. 2003, 1, 3263.
- 6
- 6aE. A. Colby, K. C. O’Brien, T. F. Jamison, J. Am. Chem. Soc. 2004, 126, 998;
- 6bE. A. Colby, K. C. O’Brien, T. F. Jamison, J. Am. Chem. Soc. 2005, 127, 4297.
- 7
- 7aB. Knapp-Reed, G. M. Mahandru, J. Montgomery, J. Am. Chem. Soc. 2005, 127, 13156;
- 7bM. R. Chaulagain, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2007, 129, 9568.
- 8We have observed the directing of regioselectivity in nickel-catalyzed coupling reactions of alkynes by a similarly positioned alkene:
- 8aK. M. Miller, T. F. Jamison, J. Am. Chem. Soc. 2004, 126, 15342;
- 8bK. M. Miller, T. Luanphaisarnnont, C. Molinaro, T. F. Jamison, J. Am. Chem. Soc. 2004, 126, 4130.
- 9
- 9aJ. Feldman, J. S. Murdzek, W. M. Davis, R. R. Schrock, Organometallics 1989, 8, 2260;
- 9bG. C. Fu, R. H. Grubbs, J. Am. Chem. Soc. 1992, 114, 7324;
- 9cA. Fürstner, K. Langemann, J. Org. Chem. 1996, 61, 3942;
- 9dA. Fürstner, O. R. Thiel, C. W. Lehmann, Organometallics 2002, 21, 331.
- 10
- 10aM. Tokunaga, J. F. Larrow, F. Kakiuchi, E. N. Jacobsen, Science 1997, 277, 936;
- 10bS. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. R. Gould, M. E. Furrow, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 1307.
- 11H. Takahashi, T. Kawakita, M. Yoshioka, S. Kobayashi, M. Ohno, Tetrahedron Lett. 1989, 30, 7095.
- 12Generation of the dialkylzinc by using Knochel’s method was critical for high stereoselectivity (3.5:1 d.r. was observed using n-C5H11MgX/ZnCl2): M. Rozema, A. Sidduri, P. Knochel, J. Org. Chem. 1992, 57, 1956.
- 13This ketone also undergoes high yielding and highly site-selective soft enolization at C4–C5 (Et3SiOTf, Et3N, 90 % yield). (Elaboration of this enolsilane to gloeosporone by Rubottom oxidation was unsuccessful.).
- 14
- 14aH. Bredereck, G. Simchen, S. Rebsdat, W. Kantlehner, P. Horn, R. Wahl, H. Hoffman, P. Grieshaber, Chem. Ber. 1968, 101, 41; for a recent use of Bredereck’s reagent in target-oriented synthesis, see:
- 14bJ. Peng, D. L. J. Clive, Org. Lett. 2007, 9, 2939.
- 15The regioselectivity was greater than 95:5 as determined by 1H NMR spectral analysis of crude 11 (the NMR yield of 11 was >95 %).
- 16
- 16aH. H. Wasserman, J. L. Ives, J. Am. Chem. Soc. 1976, 98, 7868;
- 16bH. H. Wasserman, J. L. Ives, J. Org. Chem. 1985, 50, 3573.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.