A Facile Route to Aryl Boronates: Room-Temperature, Copper-Catalyzed Borylation of Aryl Halides with Alkoxy Diboron Reagents†
Christian Kleeberg Dr.
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE (UK), Fax: (+44) 191-384-4737 http://www.dur.ac.uk/chemistry/staff/profile/?id=194
Search for more papers by this authorLi Dang
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong (China), Fax: (+852) 2358-1594 http://ihome.ust.hk/∼chzlin
Search for more papers by this authorZhenyang Lin Prof. Dr.
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong (China), Fax: (+852) 2358-1594 http://ihome.ust.hk/∼chzlin
Search for more papers by this authorTodd B. Marder Prof. Dr.
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE (UK), Fax: (+44) 191-384-4737 http://www.dur.ac.uk/chemistry/staff/profile/?id=194
Search for more papers by this authorChristian Kleeberg Dr.
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE (UK), Fax: (+44) 191-384-4737 http://www.dur.ac.uk/chemistry/staff/profile/?id=194
Search for more papers by this authorLi Dang
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong (China), Fax: (+852) 2358-1594 http://ihome.ust.hk/∼chzlin
Search for more papers by this authorZhenyang Lin Prof. Dr.
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong (China), Fax: (+852) 2358-1594 http://ihome.ust.hk/∼chzlin
Search for more papers by this authorTodd B. Marder Prof. Dr.
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE (UK), Fax: (+44) 191-384-4737 http://www.dur.ac.uk/chemistry/staff/profile/?id=194
Search for more papers by this authorC.K. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship and Dr. A. S. Batsanov (Durham) for helpful discussions concerning the X-ray diffraction studies. T.B.M. thanks AllylChem Co. Ltd. for a generous gift of B2pin2 and B2neop2 and the Royal Society (UK) for an International Outgoing Short Visit Grant. Z.L. thanks the Research Grants Council of Hong Kong (HKUST 601507) for support.
Abstract
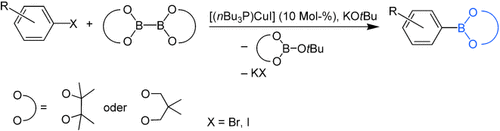
Eine einfache und effektive kupferkatalysierte Borylierung von Arylhalogeniden, einschließlich elektronenreicher und sterisch gehinderter Arylbromide, mit Alkoxydiborreagentien gelingt unter milden Bedingungen (siehe Schema). DFT-Rechnungen legen nahe, dass eine σ-Bindungsmetathese zwischen einer Kupfer-Boryl-Zwischenstufe und dem Arylhalogenid zum Arylboronat führt.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200901879_sm_miscellaneous_information.pdf1.2 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483;
- 1b Boronic Acids—Preparation, Applications in Organic Synthesis and Medicine (Ed.: ), Wiley-VCH, Weinheim, 2005;
- 1cK.-T. Wong, Y.-Y. Chien, Y.-L. Liao, C.-C. Lin, M.-Y. Chou, M.-K. Leung, J. Org. Chem. 2002, 67, 1041–1044.
- 2
- 2aT. Ishiyama, M. Murata, N. Miyaura, J. Org. Chem. 1995, 60, 7508–7510;
- 2bT. Ishiyama, Y. Itoh, T. Kitano, N. Miyaura, Tetrahedron Lett. 1997, 38, 3447–3450;
- 2cT. Ishiyama, K. Ishida, N. Miyaura, Tetrahedron 2001, 57, 9813–9816;
- 2dA. Fürstner, G. Seide, Org. Lett. 2002, 4, 541–543;
- 2eC. Xu, J.-F. Gong, M.-P. Song, Y.-J. Wu, Transition Met. Chem. 2009, 34, 175–179.
- 3
- 3aM. Murata, S. Watanabe, Y. Masuda, J. Org. Chem. 1997, 62, 6458–6459;
- 3bM. Murata, T. Oyama, S. Watanabe, Y. Masuda, J. Org. Chem. 2000, 65, 164–168;
- 3cO. Baudoin, D. Guénard, F. Guéritte, J. Org. Chem. 2000, 65, 9268–9271;
- 3dA. Wolan, M. Zaidlewicz, Org. Biomol. Chem. 2003, 1, 3274–3276.
- 4
- 4aA. B. Morgan, J. L. Jurs, J. M. Tour, J. Appl. Polym. Sci. 2000, 76, 1257–1268;
- 4bB. M. Rosen, C. Huang, V. Percec, Org. Lett. 2008, 10, 2597–2600;
- 4cD. A. Wilson, C. J. Wilson, B. M. Rosen, V. Percec, Org. Lett. 2008, 10, 4879–4882.
- 5See, for example:
- 5aJ. Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, M. R. Smith, Science 2002, 295, 305–308;
- 5bT. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 390–391;
- 5cN. Miyaura, Bull. Chem. Soc. Jpn. 2008, 81, 1535–1553;
- 5dI. A. I. Mkhalid, D. N. Coventry, D. Albesa-Jove, A. S. Batsanov, J. A. K. Howard, R. N. Perutz, Todd, B. Marder, Angew. Chem. 2006, 118, 503–505;
10.1002/ange.200503047 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 489–491;
- 5e P. Harrison, J. Morris, P. G. Steel, T. B. Marder, Synlett 2009, 147–150;
- 5fI. A. I. Mkhalid, J. C. Murphy, J. H. Barnard, T. B. Marder, J. F. Hartwig, Chem. Rev., submitted.
- 6W. Zhu, D. Ma, Org. Lett. 2006, 8, 261–263.
- 7
- 7aH. Ito, H. Yamanaka, J. Tateiwa, A. Hosomi, Tetrahedron Lett. 2000, 41, 6821–6825;
- 7bS. Mun, J.-E. Lee, J. Yun, Org. Lett. 2006, 8, 4887–4889;
- 7cJ.-E. Lee, J. Kwon, J. Yun, Chem. Commun. 2008, 733–734;
- 7dV. Lillo, A. Prieto, A. Bonet, M. M. Díaz-Requejo, J. Ramírez, P. J. Pérez, E. Fernández, Organometallics 2009, 28, 659–662.
- 8
- 8aL. Dang, Z. Lin, T. B. Marder, Organometallics 2008, 27, 4443–4454;
- 8bL. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2008, 27, 1178–1186;
- 8cL. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2007, 26, 2824–2832;
- 8dH. Zhao, L. Dang, T. B. Marder, Z. Lin, J. Am. Chem. Soc. 2008, 130, 5586–5594;
- 8eH. Zhao, Z. Lin, T. B. Marder, J. Am. Chem. Soc. 2006, 128, 15637–15643.
- 9
- 9aK. Takahashi, T. Ishiyama, N. Miyaura, J. Organomet. Chem. 2001, 625, 47–53;
- 9bK. Takahashi, T. Ishiyama, N. Miyaura, Chem. Lett. 2000, 982–983.
- 10V. Lillo, M. R. Fructos, J. Ramírez, A. A. C. Braga, F. Maseras, M. M. Díaz-Requejo, P. J. Pérez, E. Fernández, Chem. Eur. J. 2007, 13, 2614–2621.
- 11D. S. Laitar, E. Y. Tsui, J. P. Sadighi, Organometallics 2006, 25, 2405–2408.
- 12D. S. Laitar, E. Y. Tsui, J. P. Sadighi, J. Am. Chem. Soc. 2006, 128, 11036–11037.
- 13D. S. Laitar, P. Müller, J. P. Sadighi, J. Am. Chem. Soc. 2005, 127, 17196–17197.
- 14
- 14aY. Segawa, M. Yamashita, K. Nozaki, Angew. Chem. 2007, 119, 6830–6833;
10.1002/ange.200702369 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6710–6713;
- 14bT. Kajiwara, T. Terabayashi, M. Yamashita, K. Nozaki, Angew. Chem. 2008, 120, 6708–6712; Angew. Chem. Int. Ed. 2008, 47, 6606–6610.
- 15See the Supporting Information for details.
- 16
- 16aN. P. Mankad, D. S. Laitar, J. P. Sadighi, Organometallics 2004, 23, 3369–3371;
- 16bT. Ohishi, M. Nishiura, Z. Hou, Angew. Chem. 2008, 120, 5876–5879;
10.1002/ange.200801857 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5792–5795.
- 17M. R. Fructos, C. Kleeberg, T. B. Marder, unpublished results.
- 18CCDC 726766 (5 h), 726767 (5 d), 726768 (5 f), 726769 (6 a), 726770 (6 b), 726771 (6 d), and 726772 (5 m) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19
- 19aT. Ishiyama, Z. Oohashi, T. Ahiko, N. Miyaura, Chem. Lett. 2002, 780–781;
- 19bA. Giroux, Tetrahedron Lett. 2003, 44, 233–235.
- 20Y. Ma, C. Song, W. Jiang, G. Xue, J. F. Cannon, X. Wang, M. B. Andrus, Org. Lett. 2003, 5, 4635–4638.
- 21M. J. Hampden-Smith, T. T. Kodas, M. Paffett, J. D. Farr, H.-K. Shin, Chem. Mater. 1990, 2, 636–639.
- 22See, for example:
- 22aT. H. Lemmen, G. V. Goeden, J. C. Huffman, R. L. Geerts, K. G. Caulton, Inorg. Chem. 1990, 29, 3680–3685;
- 22bS. J. Lippard, J. J. Mayerle, Inorg. Chem. 1972, 11, 753–759;
- 22cM. R. Churchill, F. J. Rotella, Inorg. Chem. 1979, 18, 166–171;
- 22dR. G. Goel, A. L. Beauchamp, Inorg. Chem. 1983, 22, 395–400;
- 22eH. Schmidbaur, J. Adlkofer, K. Schwirten, Chem. Ber. 1972, 105, 3382–3388;
- 22fI. Medina, J. T. Mague, M. J. Fink, Acta Crystallogr. Sect. E 2005, 61, m 1550–m1552;
- 22gS. Díez-González, H. Kaur, F. K. Zinn, E. D. Stevens, S. P. Nolan, J. Org. Chem. 2005, 70, 4784–4796;
- 22hA. F. Wells, Z. Kristallogr., Kristallgeom., Kristallphys., Kristallchem. 1936, 94, 447–461.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.