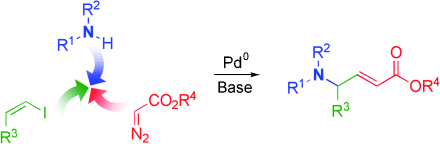
Palladium-Catalyzed Insertion of α-Diazoesters into Vinyl Halides To Generate α,β-Unsaturated γ-Amino Esters†
Romas Kudirka
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorSean K. J. Devine
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorChristopher S. Adams
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorDavid L. Van Vranken Prof.
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorRomas Kudirka
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorSean K. J. Devine
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorChristopher S. Adams
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorDavid L. Van Vranken Prof.
Department of Chemistry, University of California at Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025 (USA), Fax: (+1) 949-824-8571
Search for more papers by this authorThis work was supported by the ACS PRF 42780-AC1.
Abstract
So einfach wie auf drei zu zählen: Eine Palladium-katalysierte Dreikomponentenkupplung erzeugt in einem Schritt α,β-ungesättigte γ-Aminosäuren (siehe Schema). Die Reaktion umfasst vermutlich die Wanderung eines Vinylsubstituenten zu einem hoch elektrophilen Palladiumcarben. Anders als herkömmliche Verfahren bietet diese Synthese Zugang zu γ-Aminosäuren mit nichtnatürlichen Seitenketten.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200805483_sm_miscellaneous_information.pdf2.8 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Kudirka, D. L. Van Vranken, J. Org. Chem. 2008, 73, 3585–3588;
- 1bS. K. J. Devine, D. L. Van Vranken, Org. Lett. 2008, 10, 1909–1911;
- 1cS. K. J. Devine, D. L. Van Vranken, Org. Lett. 2007, 9, 2047–2049;
- 1dK. L. Greenman, D. L. Van Vranken, Tetrahedron 2005, 61, 6438–6441;
- 1eK. L. Greenman, D. S. Carter, D. L. Van Vranken, Tetrahedron 2001, 57, 5219–5225.
- 2
- 2aJ. E. Coleman, E. D. de Silva, K. Fangming, R. J. Andersen, T. M. Allen, Tetrahedron 1995, 51, 10653–10662;
- 2bN. Schaschke, Bioorg. Med. Chem. Lett. 2004, 14, 855–858.
- 3For a review of Michael acceptors (including α,β-unsaturated-γ-aminoesters) as protease inhibitors, see: M. M. M. Santos, R. Moreira, Mini-Rev. Med. Chem. 2007, 7, 1040–1050, and references therein.
- 4
- 4aS. Hanessian, X. Luo, R. Schaum, S. Michnick, J. Am. Chem. Soc. 1998, 120, 8569–8570;
- 4bT. Hintermann, K. Gademann, B. Jaun, D. Seebach, Helv. Chim. Acta 1998, 81, 983–1002;
- 4cS. Hanessian, X. H. Luo, R. Schaum, Tetrahedron Lett. 1999, 40, 4925–4929;
- 4dD. Seebach, M. Brenner, M. Rueping, B. Schweizer, B. Juan, Chem. Commun. 2001, 207–208;
- 4eC. Baldauf, R. Gunther, H. J. Hofmann, Helv. Chim. Acta 2003, 86, 2573–2588;
- 4ffor an example of a sheetlike structure, see: M. G. Woll, J. R. Lai, I. A. Guzei, S. J. C. Taylor, M. E. B. Smith, S. H. Gellman, J. Am. Chem. Soc. 2001, 123, 11077–11078.
- 5
- 5aJ. Jurczak, A. Golebiowski, Chem. Rev. 1989, 89, 149–164;
- 5bD. Yoo, J. S. Oh, Y. G. Kim, Org. Lett. 2002, 4, 1213–1215;
- 5cL. K. Blasdel, A. G. Myers, Org. Lett. 2005, 7, 4281–4283.
- 6C. Peng, Y. Wang, J. Wang, J. Am. Chem. Soc. 2008, 130, 1566–1567.
- 7
- 7aS. Ogoshi, T. Morimoto, K. Nishio, K. Ohe, S. Murai, J. Org. Chem. 1993, 58, 9–10;
- 7bN. Monteiro, J. Goré, B. Van Hemelryck, G. Balme, Synlett 1994, 447–449;
- 7cN. Monteiro, G. Balme, J. Org. Chem. 2000, 65, 3223–3226;
- 7dE. Fillion, N. J. Taylor, J. Am. Chem. Soc. 2003, 125, 12700–12701;
- 7eV. É. Trépanier, E. Fillion, Organometallics 2007, 26, 30–32;
- 7fJ. M. Goll, E. Fillion, Organometallics 2008, 27, 3622–3625.
- 8C. Peng, J. Cheng, J. Wang, J. Am. Chem. Soc. 2007, 129, 8708–8709.
- 9
- 9aE. Ihara, N. Haida, M. Iio, K. Inoue, Macromolecules 2003, 36, 36–41;
- 9bE. Ihara, M. Kida, M. Fujioka, N. Haida, T. Itoh, K. Inoue, J. Polym. Sci. Part A 2007, 45, 1536–1545.
- 10M. P. Di, K. S. Rein, Tetrahedron Lett. 2004, 45, 4703–4706.
- 11M. Bröring, C. D. Brandt, S. Stellwag, Chem. Commun. 2003, 2344–2345.
- 12
- 12aA. C. Albéniz, P. Espinet, R. Manrique, A. Perez-Mateo, Angew. Chem. 2002, 114, 2469–2472;
10.1002/1521-3757(20020703)114:13<2469::AID-ANGE2469>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2363–2366;10.1002/1521-3773(20020703)41:13<2363::AID-ANIE2363>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 12bA. A. Danopoulos, N. Tsoureas, J. C. Green, M. B. Hursthouse, Chem. Commun. 2003, 756–757.
- 13R. Tanikaga, J. Takeuchi, M. Takyu, A. Kaji, J. Chem. Soc. Chem. Commun. 1987, 386–387.
- 14R. Kumareswaran, Y. D. Vankar, Synth. Commun. 1998, 28, 2291–2302.
- 15L. Benati, D. Nanni, P. Spagnolo, J. Org. Chem. 1999, 64, 5132–5138.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.