A Practical Synthesis of (−)-Oseltamivir†
Nobuhiro Satoh
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorTakahiro Akiba
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorSatoshi Yokoshima Dr.
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorTohru Fukuyama Prof.
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorNobuhiro Satoh
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorTakahiro Akiba
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorSatoshi Yokoshima Dr.
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorTohru Fukuyama Prof.
Graduate School of Pharmaceutical Sciences, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5802-8694
Search for more papers by this authorThis research was supported financially in part by a grant for the 21st Century COE Program and a Grant-in-Aid (15109001 and 16073205) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We are indebted to Dr. Kunisuke Izawa of Ajinomoto Co. for an ample supply of D-phenylalanine.
Graphical Abstract
So einfach wie möglich: Das immer noch stark nachgefragte Grippemedikament (−)-Oseltamivir-Phosphat (Tamiflu; siehe Schema) wurde ausgehend von Pyridin mithilfe billiger Reagentien synthetisiert, wobei die Zahl an Reinigungsschritten konsequent gering gehalten wurde. Die Syntheseroute umfasst eine asymmetrische Diels-Alder-Reaktion, eine Bromlactonisierung, eine Hofmann-Umlagerung und eine Dominoreaktion eines Bicyclo[2.2.2]-Systems in ein Aziridinintermediat.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701754_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. U. Kim, W. Lew, M. A. Williams, H. Liu, L. Zhang, S. Swaminathan, N. Bischofberger, M. S. Chen, D. B. Mendel, C. Y. Tai, W. G. Laver, R. C. Stevens, J. Am. Chem. Soc. 1997, 119, 681.
- 2S. Abrecht, P. Harrington, H. Iding, M. Karpf, R. Trussardi, B. Wirz, U. Zutter, Chimia 2004, 58, 621.
- 3
- 3aJ. C. Rohloff, K. M. Kent, M. J. Postich, M. W. Becker, H. H. Chapman, D. E. Kelly, W. Lew, M. S. Louie, L. R. McGee, E. J. Prisbe, L. M. Schultze, R. H. Yu, L. Zhang, J. Org. Chem. 1998, 63, 4545;
- 3bM. Federspiel, R. Fischer, M. Hennig, H.-J. Mair, T. Oberhauser, G. Rimmler, T. Albiez, J. Bruhin, H. Estermann, C. Gandert, V. Göckel, S. Götzö, U. Hoffmann, G. Huber, G. Janatsch, S. Lauper, O. Röckel-Stäbler, R. Trussardi, A. G. Zwahlen, Org. Process Res. Dev. 1999, 3, 266;
- 3cM. Karpf, R. Trussardi, J. Org. Chem. 2001, 66, 2044;
- 3dP. J. Harrington, J. D. Brown, T. Foderaro, R. C. Hughes, Org. Process Res. Dev. 2004, 8, 86;
- 3eY.-Y. Yeung, S. Hong, E. J. Corey, J. Am. Chem. Soc. 2006, 128, 6310;
- 3fY. Fukuta, T. Mita, N. Fukuda, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2006, 128, 6312;
- 3gX. Cong, Z.-J. Yao, J. Org. Chem. 2006, 71, 5365;
- 3hT. Mita, N. Fukuda, F. X. Roca, M. Kanai, M. Shibasaki, Org. Lett. 2007, 9, 259;
- 3iK. Yamatsugu, S. Kamijo, Y. Suto, M. Kanai, M. Shibasaki, Tetrahedron Lett. 2007, 48, 1403;
- 3jfor a recent review of syntheses of oseltamivir, see: V. Farina, J. D. Brown, Angew. Chem. 2006, 118, 7488;
10.1002/ange.200602623 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7330.
- 4
- 4aF. W. Fowler, J. Org. Chem. 1972, 37, 1321;
- 4bR. J. Sundberg, J. D. Bloom, J. Org. Chem. 1981, 46, 4836.
- 5For enantioselective Diels–Alder reactions of dihydropyridines, see:
- 5aN. Takenaka, Y. Huang, V. H. Rawal, Tetrahedron 2002, 58, 8299;
- 5bH. Nakano, N. Tsugawa, R. Fujita, Tetrahedron Lett. 2005, 46, 5677; for diastereoselective reactions of chiral dihydropyridines, see:
- 5cM. Mehmandoust, C. Marazano, R. Singh, B. Gillet, M. Césario, J.-L. Fourrey, B. C. Das, Tetrahedron Lett. 1988, 29, 4423;
- 5dC. Marazano, S. Yannic, Y. Genisson, M. Mehmandoust, B. C. Das, Tetrahedron Lett. 1990, 31, 1995;
- 5eY. Matsumura, Y. Nakamura, T. Maki, O. Onomura, Tetrahedron Lett. 2000, 41, 7685; for diastereoselective Diels–Alder reactions of chiral acrylic acid derivatives with dihydropyridines, see:
- 5fC. Kouklovsky, A. Pouilhès, Y. Langlois, J. Am. Chem. Soc. 1990, 112, 6672;
- 5gM. M. Campbell, M. Sainsbury, P. A. Searle, G. M. Davies, Tetrahedron Lett. 1992, 33, 3181.
- 6
- 6aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 6bG. Lelais, D. W. C. MacMillan, Aldrichimica Acta 2006, 39, 79.
- 7The main by-product was benzyl (5-formylcyclohexa-2,4-dienyl)methylcarbamate, which was isolated as the corresponding alcohol after reduction of the crude mixture of aldehydes (see the Supporting Information). The exo isomer was not detected.
- 8
- 8aB. O. Lindgren, T. Nilsson, Acta Chem. Scand. 1973, 27, 888;
- 8bG. A. Kraus, M. J. Taschner, J. Org. Chem. 1980, 45, 1175.
- 9M. Sakaitani, K. Hori, Y. Ohfune, Tetrahedron Lett. 1988, 29, 2983.
- 10In an experiment with 4.5 g of 14, 93 % of the RuO2⋅n H2O used could be recovered by the addition of isopropanol to the reaction mixture followed by filtration. The recovered material could be reused and showed no sign of deterioration (see the Supporting Information). Oxone could also be used as a cooxidant (90 % over 2 steps). Quite recently, we found that n-propyl acetate, a safer solvent than dichloroethane, could be used with similar results.
- 11R. M. Moriarty, C. J. Chany II, R. K. Vaid, O. Prakash, S. M. Tuladhar, J. Org. Chem. 1993, 58, 2478.
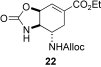
- 12The relatively low yield of 20 was attributed to the concomitant formation of oxazolidinone 22. The mixture was readily separable by chromatography on silica gel.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.





