Supramolecular Construction of Fluorescent J-Aggregates Based on Hydrogen-Bonded Perylene Dyes†
Theo E. Kaiser
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorHao Wang Dr.
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorVladimir Stepanenko
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorFrank Würthner Prof. Dr.
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorTheo E. Kaiser
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorHao Wang Dr.
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorVladimir Stepanenko
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorFrank Würthner Prof. Dr.
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4756
Search for more papers by this authorWe gratefully acknowledge the Alexander von Humboldt Foundation (postdoctoral fellowship for H.W.) and the Fonds der Chemischen Industrie for financial support of this research, and C. Z. Shao for the synthesis of the reference compound 2.
Graphical Abstract
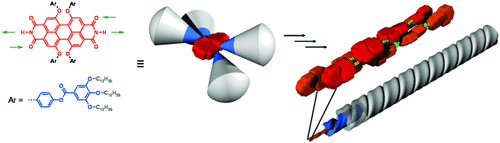
Leuchtende Nanostäbe: Die Selbstorganisation Kern-verdrillter Perylenbisimid-Fluorophore (siehe Strukturen) in unpolaren organischen Lösungsmitteln wird durch Wasserstoffbrücken gesteuert. Dieses supramolekulare Konzept führte zu eindimensionalen J-Aggregaten mit einer Fluoreszenzquantenausbeute von fast 100 %.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701139_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. E. Jelley, Nature 1936, 138, 1009–1010;
- 1bE. E. Jelley, Nature 1937, 139, 631–632;
- 1cG. Scheibe, Angew. Chem. 1936, 49, 563;
- 1dG. Scheibe, Angew. Chem. 1937, 50, 51;
10.1002/ange.19370501103 Google Scholar
- 1eE. Daltrozzo, G. Scheibe, K. Geschwind, F. Haimerl, Photogr. Sci. Eng. 1974, 18, 441–450;
- 1fH. von Berlepsch, C. Böttcher, L. Dähne, J. Phys. Chem. B 2000, 104, 8792–8799.
- 2
- 2aA. H. Herz, Adv. Colloid Interface Sci. 1977, 8, 237–298;
- 2bD. Möbius, Adv. Mater. 1995, 7, 437–444;
- 2cH. Kuhn, C. Kuhn in J-Aggregates (Ed.: ), World Scientific, Singapore, 1996, pp. 1–40;
- 2dA. Pawlik, S. Kirstein, U. De Rossi, S. Dähne, J. Phys. Chem. B 1997, 101, 5646–5651;
- 2eS. Dähne, Bunsen-Magazin 2002, 4, 81–92.
- 3
- 3aA. Ajayaghosh, S. J. George, A. P. H. J. Schenning, Top. Curr. Chem. 2005, 258, 83–118;
- 3bF. J. M. Hoeben, P. Jonkheijm, E. W. Meijer, A. P. H. Schenning, Chem. Rev. 2005, 105, 1491–1546; for a review on phosphorescent assemblies, see:
- 3cL. Bruzzone, R. Badia, M. E. Diaz Garcia, Crit. Rev. Anal. Chem. 2000, 30, 163–178.
- 4F. Würthner, Chem. Commun. 2004, 1564–1579.
- 5Some fluorescent aggregates of perylene bisimides have been reported, but they do not display J-type characteristics, such as small Stokes shifts and narrow emission bands:
- 5aD. Liu, S. De Feyter, M. Cotlet, U.-M. Wiesler, T. Weil, A. Herrmann, K. Müllen, F. C. De Schryver, Macromolecules 2003, 36, 8489–8498;
- 5bP. Yan, A. Chowdhury, M. W. Holman, D. M. Adams, J. Phys. Chem. B 2005, 109, 724–730;
- 5cZ. Chen, V. Stepanenko, V. Dehm, P. Prins, L. D. A. Siebbeles, J. Seibt, P. Marquetand, V. Engel, F. Würthner, Chem. Eur. J. 2007, 13, 436–449.
- 6
- 6aA. R. Holzwarth, K. Schaffner, Photosynth. Res. 1994, 41, 225–233;
- 6bG. McDermott, S. M. Prince, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Papiz, R. J. Cogdell, N. W. Isaacs, Nature 1995, 374, 517–521;
- 6cT. Pullerits, V. Sundström, Acc. Chem. Res. 1996, 29, 381–389;
- 6dT. S. Balaban, H. Tamiaki, A. R. Holzwarth, Top. Curr. Chem. 2005, 258, 1–38.
- 7V. I. Prokhorenko, D. B. Steensgard, A. R. Holzwarth, Biophys. J. 2000, 79, 2105–2120.
- 8
- 8aR. Takahashi, Y. Kobuke, J. Am. Chem. Soc. 2003, 125, 2372–2373;
- 8bT. Yamaguchi, T. Kimura, H. Matsuda, T. Aida, Angew. Chem. 2004, 116, 6510–6515;
10.1002/ange.200461431 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6350–6355;
- 8cJ. A. A. W. Elemans, R. van Hameren, R. J. M. Nolte, A. E. Rowan, Adv. Mater. 2006, 18, 1251–1266.
- 9F. Würthner, Pure Appl. Chem. 2006, 78, 2341–2349.
- 10V. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H.-W. Spiess, S. D. Hudson, H. Duan, Nature 2002, 417, 384–387.
- 11J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995.
10.1002/3527607439 Google Scholar
- 12The perylene bisimide 1 was characterized by NMR spectroscopy, mass spectrometry, and elemental analysis: m.p. 246–247 °C; 1H NMR (CDCl3, 400.13 MHz, 300 K, TMS): δ=8.31 (s, 2 H, NH), 8.17 (s, 4 H, Hpery), 7.33 (s, 8 H, Haryl), 7.15–7.10 (m, 8 H, Haryl), 6.97–6.92 (m, 8 H, Haryl), 4.02–3.93 (m, 24 H, OCH2), 1.85–1.62 (m, 24 H, CH2), 1.55–1.35 (m, 24 H, CH2), 1.35–1.10 (m, 192 H, CH2), 0.84–0.78 ppm (m, 36 H, CH3); MS (MALDI-TOF, positive mode, DCTB): m/z calcd for C220H332N2O28: 3450.46 [M+2 H]+; found: 3450.40; UV/Vis (CH2Cl2): λmax (ε)=570 (41 600), 533 (26 800), 444 nm (19 000 m−1 cm−1); fluorescence (CH2Cl2, λex=535 nm): λmax=602 nm, Φfl=0.93±0.01; elemental analysis: calcd (%) for C220H330N2O28⋅H2O (3466.46): C 76.17, H 9.65, N 0.81; found: C 76.04, H 9.55, N 0.89.
- 13Transition dipole moments were calculated according to a reported method: W. Liptay, R. Wortmann, H. Schaffrin, O. Burkhard, W. Reitinger, N. Detzer, Chem. Phys. 1988, 120, 429–438. The molar extinction coefficient and the transition dipole moment for the aggregate are given as the values per aggregate-bound monomeric unit.
- 14Z. Chen, U. Baumeister, C. Tschierske, F. Würthner, Chem. Eur. J. 2007, 13, 450–465.
- 15R. P. Sijbesma, F. H. Beijer, L. Brunsveld, B. J. B. Folmer, J. H. K. K. Hirschberg, R. F. M. Lange, J. K. L. Lowe, E. W. Meijer, Science 1997, 278, 1601–1604.
- 16M. Kasha, H. R. Rawls, M. A. El-Bayoumi, Pure Appl. Chem. 1965, 11, 371–392.
- 17For a discussion, see the Supporting Information.
- 18For further details, see the Supporting Information.
- 19
- 19aR. M. Jones, L. D. Lu, R. Helgeson, T. S. Bergstedt, D. W. McBranch, D. G. Whitten, Proc. Natl. Acad. Sci. USA 2001, 98, 14769–14772;
- 19bA. Rose, Z. G. Zhu, C. F. Madigan, T. M. Swager, V. Bulovic, Nature 2005, 434, 876–879.
- 20Previously reported fluorescence quantum yields for cyanine-dye J-aggregates are about 40 % at room temperature and 70 % at 77 K; see:
- 20aI. G. Scheblykin, O. P. Varnavsky, M. M. Bataiev, O. Sliusarenko, M. van der Auweraer, A. G. Vitukhnovsky, Chem. Phys. Lett. 1998, 298, 341–350;
- 20bI. G. Scheblykin, M. Bataiev, M. van der Auweraer, A. G. Vitukhnovsky, Chem. Phys. Lett. 2000, 316, 37–44.
- 21A coherent size N of three to four molecules was estimated by an alternative approach on the basis of the fwhm values of the corresponding 0–0 vibronic transitions according to the equation Δνfwhm,mon/Δνfwhm,agg=
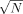 (G. Busse, B. Frederichs, N. K. Petrov, S. Techert, Phys. Chem. Chem. Phys. 2004, 6, 3309–3314).
(G. Busse, B. Frederichs, N. K. Petrov, S. Techert, Phys. Chem. Chem. Phys. 2004, 6, 3309–3314).
- 22S. S. Lampoura, C. Spitz, S. Dähne, J. Knoester, K. Duppen, J. Phys. Chem. B 2002, 106, 3103–3111.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.