Iron-Catalyzed Alkylations of Aromatic Grignard Reagents†
Gérard Cahiez Prof.
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorVanessa Habiak
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorChristophe Duplais
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorAlban Moyeux
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorGérard Cahiez Prof.
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorVanessa Habiak
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorChristophe Duplais
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorAlban Moyeux
Laboratoire de Synthèse Organique Sélective et de Chimie Organométallique (SOSCO), UMR 8123 CNRS-UCP-ESCOM, 5 Mail Gay-Lussac, Neuville s/Oise, 95092 Cergy-Pontoise, France, Fax: (+33) 1-3425-7383
Search for more papers by this authorWe thank the CNRS and the Ecole Supérieure de Chimie Organique et Minérale (ESCOM) for their financial support as well as the Ministère de l'Education Nationale et de la Recherche for its financial support and a grant to A.M. The Fondation de France is acknowledged for a grant to C.D. and V.H.
Graphical Abstract
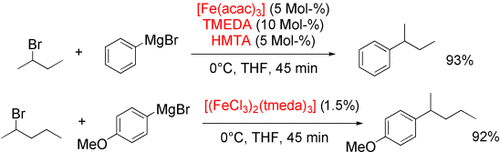
Nicht zum alten Eisen: Zwei effiziente eisenkatalysierte Kreuzkupplungen von Aryl-Grignard-Reagentien mit Alkylbromiden eignen sich für die Anwendung im großen Maßstab. Das erste Verfahren nutzt Eisenacetylacetonat und einen kooperativen Effekt der Liganden N,N,N′,N′-Tetramethylethylendiamin (TMEDA) und Hexamethylentetraamin (HMTA), im zweiten dient [(FeCl3)2(tmeda)3] als Katalysator.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z700742_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Cahiez, P. Y. Chavant, E. Metais, Tetrahedron Lett. 1992, 33, 5245;
- 1bG. Cahiez, S. Marquais, Tetrahedron Lett. 1996, 37, 1773;
- 1cG. Cahiez, H. Avedissian, Synthesis 1998, 1199;
- 1dC. Duplais, F. Bures, T. Korn, I. Sapountzis, G. Cahiez, P. Knochel, Angew. Chem. 2004, 116, 3028;
10.1002/ange.200453696 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2968.
- 2For exhaustive reviews on iron-mediated coupling reactions, see:
- 2aA. Fürstner, R. Martin, Chem. Lett. 2005, 34, 624;
- 2bC. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217;
- 2cH. Shinokubo, K. Oshima, Eur. J. Org. Chem. 2004, 2071.
- 3aT. Nagano, T. Hayashi, Org. Lett. 2004, 6, 1297;
- 3bR. B. Bedford, D. W. Bruce, R. M. Frost, J. W. Goodby, M. Hird, Chem. Commun. 2004, 2822;
- 3cR. B. Bedford, M. Betham, D. W. Bruce, A. A. Danopoulos, R. M. Frost, M. Hird, J. Org. Chem. 2006, 71, 1104;
- 3dR. B. Bedford, D. W. Bruce, R. M. Frost, M. Hird, Chem. Commun. 2005, 4161;
- 3eR. Martin, A. Fürstner, Angew. Chem. 2004, 116, 4045; Angew. Chem. Int. Ed. 2004, 43, 3955;
- 3fM. Nakamura, K. Matsuo, S. Ito, E. Nakamura, J. Am. Chem. Soc. 2004, 126, 3686.
- 4See reference [3f] and Table S2 in the Supporting Information.
- 5aI. D. Hills, M. R. Netherton, G. C. Fu, Angew. Chem. 2003, 115, 5927;
10.1002/ange.200352858 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5749. For general reviews on the use of alkyl halides to perform cross-coupling reactions, see:
- 5bA. C. Frisch, M. Beller, Angew. Chem. 2005, 117, 680; Angew. Chem. Int. Ed. 2005, 44, 674;
- 5cM. R. Netherton, G. C. Fu, Adv. Synth. Catal. 2004, 346, 1525;
- 5dD. J. Cárdenas, Angew. Chem. 2003, 115, 398;
10.1002/ange.200390091 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 384, and references therein.
- 6The mechanism is based on the Fe0/FeII couple. A similar catalytic cycle can also be written by using a FeI/FeIII couple (see S. M. Neumann, J. K. Kochi, J. Org. Chem. 1975, 40, 599 and R. S. Smith, J. K. Kochi, J. Org. Chem. 1976, 41, 502) or a Fe−II/Fe0 couple as recently proposed ( A. Fürstner, A. Leitner, M. Méndez, H. Krause, J. Am. Chem. Soc. 2002, 124, 13856; A. Fürstner, A. Leitner, Angew. Chem. 2002, 114, 632; Angew. Chem. Int. Ed. 2002, 41, 609). Iron ate complexes have been isolated recently:
- 6aA. Fürstner, H. Krause, C. W. Lehman, Angew. Chem. 2006, 118, 454;
10.1002/ange.200502859 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 440;
- 6bP. J. Alonso, A. B. Arauzo, J. Forniés, M. A. García-Montforte, A. Martín, J. I. Martínez, B. Menjón, C. Rillo, J. J. Sáiz-Garitaonandia, Angew. Chem. 2006, 118, 6859;
10.1002/ange.200601774 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6707.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.