3-Boryl-2,2′-bithiophene as a Versatile Core Skeleton for Full-Color Highly Emissive Organic Solids†
Atsushi Wakamiya Dr.
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorKenji Mori
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorShigehiro Yamaguchi Prof. Dr.
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorAtsushi Wakamiya Dr.
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorKenji Mori
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorShigehiro Yamaguchi Prof. Dr.
Department of Chemistry, Graduate School of Science, Nagoya University, and SORST, Japan Science and Technology Agency, Chikusa, Nagoya 464-8602, Japan, Fax: (+81) 52-789-5947
Search for more papers by this authorThis work was partially supported by SORST, JST, and the TOYOTA Motor Co.
Graphical Abstract
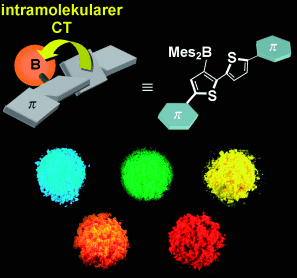
Farbenfroh: Durch Einstellen der Elektronendonoreigenschaften des π-konjugierten Bithiophen-Gerüsts wurden intensiv emittierende Festkörper erhalten, deren Maxima einen großen Teil des sichtbaren Spektrums abdecken (siehe Bild). Eine tiefrote Fluoreszenz mit großer Stokes-Verschiebung um 200 nm resultiert aus einem intramolekularen Charge-Transfer(CT)-Übergang vom verdrillten Bithiophen auf das Borzentrum.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z604935_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Examples of highly emissive organic solids:
- 1aC.-T. Chen, C.-L. Chiang, Y.-C. Lin, L.-H. Chan, C.-H. Huang, Z.-W. Tsai, C.-T. Chen, Org. Lett. 2003, 5, 1261;
- 1bH. Langhals, O. Krotz, K. Polborn, P. Mayer, Angew. Chem. 2005, 117, 2479;
10.1002/ange.200461610 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2427;
- 1cS. H. Lee, B.-B. Jang, Z. H. Kafafi, J. Am. Chem. Soc. 2005, 127, 9071;
- 1dY. Kim, J. Bouffard, S. E. Kooi, T. M. Swager, J. Am. Chem. Soc. 2005, 127, 13726;
- 1eZ. Xie, B. Yang, F. Li, G. Cheng, L. Liu, G. Yang, H. Xu, L. Ye, M. Hanif, S. Liu, D. Ma, Y. Ma, J. Am. Chem. Soc. 2005, 127, 14152;
- 1fA. Hayer, V. Halleux, A. Köhler, A. El-Garoughy, E. W. Meijer, J. Barberá, J. Tant, J. Levin, M. Lehmann, J. Gierschner, J. Cornil, Y. H. Geerts, J. Phys. Chem. B 2006, 110, 7653.
- 2C.-H. Zhao, A. Wakamiya, Y. Inukai, S. Yamaguchi, J. Am. Chem. Soc. 2006, 128, 15934.
- 3Reviews on organoboron materials:
- 3aC. D. Entwistle, T. B. Marder, Angew. Chem. 2002, 114, 3051;
10.1002/1521-3757(20020816)114:16<3051::AID-ANGE3051>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2927;10.1002/1521-3773(20020816)41:16<2927::AID-ANIE2927>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 3bC. D. Entwistle, T. B. Marder, Chem. Mater. 2004, 16, 4574.
- 4aP. J. Grisdale, J. L. R. Williams, M. E. Glogowski, B. E. Babb, J. Org. Chem. 1971, 36, 544;
- 4bJ. C. Doty, B. Babb, P. J. Grisdale, M. Glogowski, J. L. R. Williams, J. Organomet. Chem. 1972, 38, 229.
- 5aZ. Yuan, N. J. Taylor, T. B. Marder, I. D. Williams, S. K. Kurtz, L.-T. Cheng, J. Chem. Soc. Chem. Commun. 1990, 1489;
- 5bZ. Yuan, N. J. Taylor, Y. Sun, T. B. Marder, I. D. Williams, L.-T. Cheng, J. Organomet. Chem. 1993, 449, 27;
- 5cZ. Yuan, N. J. Taylor, R. Ramachandran, T. B. Marder, Appl. Organomet. Chem. 1996, 10, 305;
- 5dZ. Yuan, J. C. Collings, N. J. Taylor, T. B. Marder, C. Jardin, J.-F. Halet, J. Solid State Chem. 2000, 154, 5;
- 5eT. B. Marder et al., Chem. Eur. J. 2006, 12, 2758, see the Supporting Information.
- 6aM. Lequan, R. M. Lequan, C. K. Ching, J. Mater. Chem. 1991, 1, 997;
- 6bC. Branger, M. Lequan, R. M. Lequan, M. Barzoukas, A. Fort, J. Mater. Chem. 1996, 6, 555;
- 6cC. Branger, M. Lequan, R. M. Lequan, M. Large, F. Kajzar, Chem. Phys. Lett. 1997, 272, 265.
- 7aT. Noda, Y. Shirota, J. Am. Chem. Soc. 1998, 120, 9714;
- 7bT. Noda, H. Ogawa, Y. Shirota, Adv. Mater. 1999, 11, 283;
- 7cY. Shirota, M. Kinoshita, T. Noda, K. Okumoto, T. Ohara, J. Am. Chem. Soc. 2000, 122, 11021;
- 7dH. Doi, M. Kinoshita, K. Okumoto, Y. Shirota, Chem. Mater. 2003, 15, 1080.
- 8aS. Yamaguchi, T. Shirasaka, K. Tamao, Org. Lett. 2000, 2, 4129;
- 8bS. Yamaguchi, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2001, 123, 11372;
- 8cS. Yamaguchi, T. Shirasaka, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2002, 124, 8816;
- 8dY. Kubo, M. Yamamoto, M. Ikeda, M. Takeuchi, S. Shinkai, S. Yamaguchi, K. Tamao, Angew. Chem. 2003, 115, 2082;
10.1002/ange.200250788 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2036.
- 9aZ.-Q. Liu, Q. Fang, D. Wang, G. Xue, W.-T. Yu, Z.-S. Shao M.-H. Jiang, Chem. Commun. 2002, 2900;
- 9bZ.-Q. Liu, Q. Fang, D.-X. Cao, D. Wang, G.-B. Xu, Org. Lett. 2004, 6, 2933.
- 10aW.-L. Jia, D. Song, S. Wang, J. Org. Chem. 2003, 68, 701;
- 10bW.-L. Jia, D.-R. Bai, T. McCormick, Q.-D. Liu, M. Motala, R.-Y. Wang, C. Seward, Y. Tao, S. Wang, Chem. Eur. J. 2004, 10, 994;
- 10cW. L. Jia, M. J. Moran, Y. Y. Yuan, Z. H. Lu, S. Wang, J. Mater. Chem. 2005, 15, 3326;
- 10dW.-L. Jia, X. D. Feng, D. R. Bai, Z. H. Lu, S. Wang, G. Vamvounis, Chem. Mater. 2005, 17, 164;
- 10eQ.-D. Liu, M. S. Mudadu, R. Thummel, Y. Tao, S. Wang, Adv. Funct. Mater. 2005, 15, 143;
- 10fX. Y. Liu, D. R. Bai, S. Wang, Angew. Chem. 2006, 118, 5601; Angew. Chem. Int. Ed. 2006, 45, 5475.
- 11aA. Sundararaman, M. Victor, R. Varughese, F. Jäkle, J. Am. Chem. Soc. 2005, 127, 13748;
- 11bK. Parab, K. Venkatasubbaiah, F. Jäkle, J. Am. Chem. Soc. 2006, 128, 12879;
- 11cY. Qin, I. Kiburu, S. Shah, F. Jäkle, Org. Lett. 2006, 8, 5227.
- 12R. Stahl, C. Lambert, C. Kaiser, R. Wortmann, R. Jakober, Chem. Eur. J. 2006, 12, 2358.
- 13
- 13aT. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 4685;
- 13bV. Farina, B. Krishnan, J. Am. Chem. Soc. 1991, 113, 9585.
- 14Crystal data for 1 and 8: see the Supporting Information. CCDC-629218 (1) and CCDC-629219 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15A similar small solvent dependence was also observed for π-extended 6 and 7, thus indicating that this is a general characteristic for the present π systems: see the Supporting Information.
- 16
- 16aK. R. Justin Thomas, J. T. Lin, M. Velusamy, Y.-T. Tao, C.-H. Chuen, Adv. Funct. Mater. 2004, 14, 83;
- 16bK. R. Justin Thomas, J. T. Lin, Y.-T. Tao, C.-H. Chuen, Adv. Mater. 2002, 14, 822;
- 16cS. Kato, T. Matsumoto, T. Ishi-i, T. Thiemann, M. Shigeiwa, H. Gorohmaru, S. Maeda, Y. Yamashita, S. Mataka, Chem. Commun. 2004, 2342;
- 16dS. Kato, T. Matsumoto, M. Shigeiwa, H. Goroumaru, S. Maeda, T. Ishi-i, S. Mataka, Chem. Eur. J. 2006, 12, 2303;
- 16eQ. Fang, B. Xu, B. Jiang, H. Fu, X. Chen, A. Cao, Chem. Commun. 2005, 1468;
- 16fJ. Jacob, S. Sax, T. Piok, E. J. W. List, A. C. Grimsdale, K. Müllen, J. Am. Chem. Soc. 2004, 126, 6987;
- 16gJ. Qu, C. Kohl, M. Pottek, K. Müllen, Angew. Chem. 2004, 116, 1554; Angew. Chem. Int. Ed. 2004, 43, 1528;
- 16hG. Barbarella, L. Favaretto, G. Sotgiu, M. Zambianchi, A. Bongini, C. Arbizzani, M. Mastragostino, M. Anni, G. Gigli, R. Cingolani, J. Am. Chem. Soc. 2000, 122, 11971;
- 16iS. Beaupré, M. Leclerc, Adv. Funct. Mater. 2002, 12, 192;
- 16jW.-C. Wu, H.-C. Yeh, L.-H. Chan, C.-T. Chen, Adv. Mater. 2002, 14, 1072.
- 17aQ. Hou, Y. Xu, W. Yang, M. Yuan, J. Peng, Y. Cao, J. Mater. Chem. 2002, 12, 2887;
- 17bR. Yang, R. Tian, J. Yan, Y. Zhang, J. Yang, Q. Hou, W. Yang, C. Zhang, Y. Cao, Macromolecules 2005, 38, 244;
- 17cJ. Yang, C. Jiang, Y. Zhang, R. Yang, W. Yang, Q. Hou, Y. Cao, Macromolecules 2004, 37, 1211;
- 17dQ. Peng, Z.-Y. Lu, Y. Huang, M.-G. Xie, S.-H. Han, J.-B. Peng, Y. Cao, Macromolecules 2004, 37, 260;
- 17eX. H. Zhang, B. J. Chen, X. Q. Lin, O. Y. Wong, C. S. Lee, H. L. Kwong, S. T. Lee, S. K. Wu, Chem. Mater. 2001, 13, 1565;
- 17fL.-H. Chan, Y.-D. Lee, C.-T. Chen, Macromolecules 2006, 39, 3262.
- 18
- 18aD. W. Brousmiche, J. M. Serin, J. M. J. Fréchet, G. S. He, T.-C. Lin, S. J. Chung, P. N. Prasad, J. Am. Chem. Soc. 2003, 125, 1448;
- 18bC. Hippius, F. Schlosser, M. O. Vysotsky, V. Böhmer, F. Würthner, J. Am. Chem. Soc. 2006, 128, 3870.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.