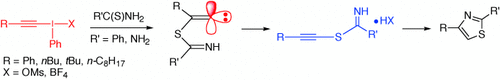
Thiazole Synthesis by Cyclocondensation of 1-Alkynyl(phenyl)-λ3-iodanes with Thioureas and Thioamides†
Kazunori Miyamoto
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorYoshio Nishi Dr.
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorMasahito Ochiai Prof.
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorKazunori Miyamoto
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorYoshio Nishi Dr.
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorMasahito Ochiai Prof.
Faculty of Pharmaceutical Sciences, University of Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Fax: (+81) 88-633-9504
Search for more papers by this authorWe gratefully acknowledge the Ministry of Education, Culture, Sports, Science, and Technology of Japan for financial support in the form of a grant.
Graphical Abstract
Die Isolierung und intramolekulare Cyclisierung von (S)-(1-Alkinyl)isothiouronium- und (S)-(1-Alkinyl)thiobenzimidoniumsalzen lassen Schlüsse auf den Mechanismus der Eintopfsynthese von 2,4-disubstituierten Thiazolen zu (siehe Schema; Ms=Methansulfonyl): Michael-Addition von Schwefelnucleophilen an hypervalente Iodane und anschließende 1,2-Umlagerung von Sulfenylgruppen in den gebildeten Alkylidencarbenen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z502438_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Wipf, S. Venkatraman, J. Org. Chem. 1996, 61, 8004.
- 2
- 2aZ.-C. Chen, P.-F. Zhang, Synthesis 2001, 358;
- 2bZ.-C. Chen, P.-F. Zhang, J. Heterocycl. Chem. 2001, 38, 503.
- 3
- 3aP. Brookes, A. T. Fuller, J. Walker, J. Chem. Soc. 1957, 689;
- 3bK. Shin-ya, K. Wierzba, K. Matsuo, T. Ohtani, Y. Yamada, K. Furihata, Y. Hayakawa, H. Seto, J. Am. Chem. Soc. 2001, 123, 1262;
- 3cL. J. Perez, D. J. Faulkner, J. Nat. Prod. 2003, 66, 247;
- 3dP. Wipf, Chem. Rev. 1995, 95, 2115;
- 3eZ. Jin, Nat. Prod. Rep. 2005, 22, 196.
- 4This [3,3]-sigmatropic rearrangement mechanism was also reported along with the synthesis of 2-mercaptothiazoles and selenazoles.[2]
- 5M. Ochiai, Y. Takaoka, Y. Nagao, J. Am. Chem. Soc. 1988, 110, 6565.
- 6P. J. Stang, V. V. Zhdankin, J. Am. Chem. Soc. 1991, 113, 4571.
- 7B. L. Williamson, P. Murch, D. R. Fischer, P. J. Stang, Synlett 1993, 858.
- 8Z.-D. Liu, Z.-C. Chen, J. Org. Chem. 1993, 58, 1924.
- 9
- 9aT. Kitamura, R. Furuki, L. Zheng, T. Fujimoto, H. Taniguchi, Chem. Lett. 1992, 2241;
- 9bD. R. Fischer, B. L. Williamson, P. J. Stang, Synlett 1992, 535.
- 10
- 10aM. Ochiai, M. Kunishima, S. Tani, Y. Nagao, J. Am. Chem. Soc. 1991, 113, 3135;
- 10bR. R. Tykwinski, J. A. Whiteford, P. J. Stang, J. Chem. Soc. Chem. Commun. 1993, 1800;
- 10cB. L. Williamson, R. R. Tykwinski, P. J. Stang, J. Am. Chem. Soc. 1994, 116, 93;
- 10dT. Kosaka, T. Bando, K. Shishido, Chem. Commun. 1997, 1167;
- 10eK. S. Feldman, M. L. Wrobleski, Org. Lett. 2000, 2, 2603;
- 10fK. S. Feldman, J. C. Saunders, J. Am. Chem. Soc. 2002, 124, 9060.
- 11(S)-(Ethylthioethynyl)isothiouronium chloride was prepared from ethylthiochloroacetylene by reaction with thiourea in 44 % yield; see: S. G. Dyachkova, N. K. Gusarova, E. A. Nikitina, L. I. Larina, L. M. Sinegovskaya, A. V. Abramov, B. A. Trofimov, Russ. J. Gen. Chem. 2001, 71, 1721.
- 12Crystal data for 9-MsOH (R=Ph, R′=NH2): C10H12N2O3S2, colorless block, dimensions 0.50×0.50×0.30 mm3, orthorhombic, P212121 (No. 19), a=6.271(1), b=12.077(2), c=16.638(3) Å, V=1260.0(4) Å3, Z=4, ρcalcd=1.436 g cm−3. Data collected on a Rigaku RAXIS-RAPID imaging plate diffractometer with MoKα radiation (λ=0.71075 Å) at T=93 K, 2θmax=54.9°, 12160 reflections measured, of which 12112 unique (Rint=0.017), μ=4.20 cm−1. R=0.026, Rw=0.025. For 10: C15H20BF4NS, colorless block, dimensions 0.30×0.30×0.20 mm3, monoclinic, P21/c (No. 14), a=11.756(5), b=23.188(8), c=12.865(5) Å, β=105.24(3)°, V=3383(2) Å3, Z=8, ρcalcd=1.308 g cm−3. MoKα radiation (λ=0.71075 Å) at T=93 K, 2θmax=54.8°, 31313 reflections measured, of which 30 889 unique (Rint=0.076), μ=2.24 cm−1. R=0.122, Rw=0.145. CCDC-269174 (9-MsOH) and -269175 (10) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13T. N. Komarova, A. S. Nakhmanovich, T. E. Glotova, V. N. Elokhina, A. L. Albanov, V. A. Lopyrev, Russ. Chem. Bull. 1997, 46, 195.
- 14M. Ochiai, M. Kunishima, K. Sumi, Y. Nagao, E. Fujita, M. Arimoto, H. Yamaguchi, Tetrahedron Lett. 1985, 26, 4501.
- 15M. Ochiai, Y. Nishi, S. Hashimoto, Y. Tsuchimoto, D.-W. Chen, J. Org. Chem. 2003, 68, 7887.
- 16G. Vernin, S. Treppendahl, J. Metzger, Helv. Chim. Acta 1977, 60, 284.
- 17Reviews:
- 17aM. Ochiai in Topics in Current Chemistry, Vol. 224 (Ed.: ), Springer, Berlin, 2003, p. 5;
- 17bP. J. Stang, J. Org. Chem. 2003, 68, 2997;
- 17cV. V. Zhdankin, P. J. Stang, Tetrahedron 1998, 54, 10927;
- 17dG. F. Koser in The Chemistry of Halides, Pseudo-halides, and Azides, Supplement D2 (Eds.: ), Wiley, New York, 1995, p. 1173;
10.1002/047002349X.ch21 Google Scholar
- 17eA. Varvoglis, The Organic Chemistry of Polycoordinated Iodine, VCH, Weinheim, 1992.
- 18However, hard nucleophiles, such as 2-lithiofuran and 2-lithiothiophene, attack the positively charged iodine of 1-alkynyl-λ3-iodanes; see: A. J. Margida, G. F. Koser, J. Org. Chem. 1984, 49, 4703.
- 19M. Ochiai, S. Yamamoto, T. Suefuji, D.-W. Chen, Org. Lett. 2001, 3, 2753.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.