Deracemization of Quaternary Stereocenters by Pd-Catalyzed Enantioconvergent Decarboxylative Allylation of Racemic β-Ketoesters†
Justin T. Mohr
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorDouglas C. Behenna
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorAndrew M. Harned Dr.
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorBrian M. Stoltz Prof.
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorJustin T. Mohr
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorDouglas C. Behenna
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorAndrew M. Harned Dr.
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorBrian M. Stoltz Prof.
The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Boulevard, MC 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorThe authors wish to thank the Fannie and John Hertz Foundation (predoctoral fellowship to D.C.B.), the NIH (postdoctoral fellowship to A.M.H.), Research Corporation, the Camille and Henry Dreyfus Foundation, Merck, Pfizer, and Lilly for financial support, and Prof. D. A. Dougherty for helpful discussions.
Graphical Abstract
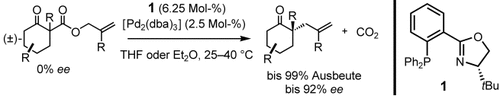
Stereochemische Alchemie! Aus racemischen Allyl-β-ketoestern lassen sich regiokontrolliert Enolate erzeugen, wobei derselbe Katalysator sowohl den Abbau (C-C-Bindungsbruch) als auch den selektiven Aufbau eines Stereozentrums (C-C-Verknüpfung) vermittelt. Auf diese Art wurden mehrere quartäre Kohlenstoffstereozentren in einer einzigen Kaskadenreaktion gebildet (siehe Schema).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z502018_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aR. Noyori, M. Tokunaga, M. Kitamura, Bull. Chem. Soc. Jpn. 1995, 68, 36–56;
- 1bH. Stecher, K. Faber, Synthesis 1997, 1–16;
- 1cF. F. Huerta, A. B. E. Minidis, J.-E. Bäckvall, Chem. Soc. Rev. 2001, 30, 321–331;
- 1dH. Pellissier, Tetrahedron 2003, 59, 8291–8327.
- 2For representative stereomutative examples, see:
- 2aRef. [1];
- 2bA. Pfaltz,M. Lautens in Comprehensive Asymmetric Catalysis, Vol. 2 (Eds: ), Springer, New York, 1999, pp. 833–884;
- 2cB. M. Trost, R. C. Bunt, R. C. Lemoine, T. L. Calkins, J. Am. Chem. Soc. 2000, 122, 5968–5976.
- 3The term stereoablation describes the conversion of a chiral molecule to an achiral molecule (e.g., Figure 1, pathway II, (±)-A→C). Ablation from the Oxford English Dictionary meaning “the action or process of carrying away or removing; removal”.
- 4For representative stereoablative examples, see:
- 4aT. Hamada, A. Chieffi, J. Åhman, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1261–1268;
- 4bN. Mase, F. Tanaka, C. F. Barbas III, Angew. Chem. 2004, 116, 2474–2477;
10.1002/ange.200353546 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2420—2423;
- 4cC. Fischer, G. C. Fu, J. Am. Chem. Soc. 2005, 127, 4594–4595;
- 4dD. Magdziak, G. Lalic, H. M. Lee, K. C. Fortner, A. D. Aloise, M. D. Shair, J. Am. Chem. Soc. 2005, 127, 7284–7285.
- 5To the best of our knowledge, there are no examples of the catalytic asymmetric synthesis of quaternary carbon stereocenters from racemates with quaternary stereocenters.
- 6A diastereoselective example of such a process has been recently reported, see: K. Xu, G. Lalic, S. M. Sheehan, M. D. Shair, Angew. Chem. 2005, 117, 2299–2301; Angew. Chem. Int. Ed. 2005, 44, 2259–2261.
- 7D. C. Behenna, B. M. Stoltz, J. Am. Chem. Soc. 2004, 126, 15044–15045.
- 8
- 8aJ. Tsuji, I. Minami, Acc. Chem. Res. 1987, 20, 140–145;
- 8bJ. Tsuji, Proc. Jpn. Acad. Ser. B 2004, 80, 349–358.
- 9
- 9aG. Helmchen, A. Pfaltz, Acc. Chem. Res. 2000, 33, 336–345;
- 9bJ. M. J. Williams, Synlett 1996, 705–710, and references therein.
- 10Subsequent to our initial report, a similar transformation using a bisphosphine ligand was reported, see: B. M. Trost, J. Xu, J. Am. Chem. Soc. 2005, 127, 2846–2847.
- 11An elegant alternative catalytic asymmetric alkylation of cyclic ketones for the synthesis of quaternary stereocenters has been recently described, see: A. G. Doyle, E. N. Jacobsen, J. Am. Chem. Soc. 2005, 127, 62–63.
- 12See Supporting Information for details.
- 13This hypothesis has been experimentally supported for nonenantioselective systems, see:
- 13aJ. Tsuji, T. Yamada, I. Minami, M. Yuhara, M. Nisar, I. Shimizu, J. Org. Chem. 1987, 52, 2988–2995;
- 13bJ.-F. Detalle, A. Riahi, V. Steinmetz, F. Hénin, J. Muzart, J. Org. Chem. 2004, 69, 6528–6532.
- 14For an excellent review on metal-catalyzed decarboxylation, see: J. A. Tunge, E. C. Burger, Eur. J. Org. Chem. 2005, 9, 1715–1726.
- 15
- 15aL. N. Mander, S. P. Sethi, Tetrahedron Lett. 1983, 24, 5425–5428;
- 15bD. M. X. Donnelly, J.-P. Finet, B. A. Rattigan, J. Chem. Soc. Perkin Trans. 1 1993, 1729–1735.
- 16
- 16aJ. A. May, B. M. Stoltz, J. Am. Chem. Soc. 2002, 124, 12426–12427;
- 16bR. Sarpong, J. T. Su, B. M. Stoltz, J. Am. Chem. Soc. 2003, 125, 13624–13625.
- 17When PPh3 was used as the ligand for the conversion of (±)-13 into (±)-14 and the meso isomer of 14 (not shown), a diastereomeric ratio of 70:30 was observed.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.