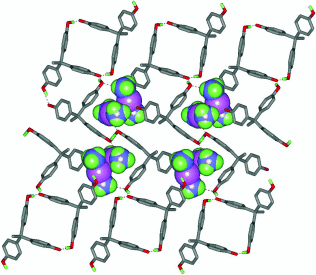
An Unstable Ligand-Unsupported CuI Dimer Stabilized in a Supramolecular Framework†
Shao-Liang Zheng Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorMarc Messerschmidt Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorPhilip Coppens Prof. Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorShao-Liang Zheng Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorMarc Messerschmidt Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorPhilip Coppens Prof. Dr.
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260–3000, USA, Fax: (+1) 716-645-6948
Search for more papers by this authorThis work was supported by the Petroleum Research Fund of the American Chemical Society (PRF32638AC3) and the National Science Foundation (CHE0236317). The authors thank Dr. Irina Novozhilova and Dr. Anatoliy Volkov for help with the theoretical calculations.
Graphical Abstract
Stabile Umgebung: Das [{Cu(NH3)2}2]2+-Dimer mit schwacher Cuprophilie wird in den Hohlräumen eines supramolekularen Gerüsts stabilisiert (siehe Struktur). Diese Stabilisierung eines sonst unbeständigen CuI-Dimers in einer supramolekularen Matrix bietet eine neuartige Möglichkeit, das isolierte Dimer systematisch – im Grundzustand und im angeregten Zustand – zu untersuchen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z501154_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. Schmidbaur, Chem. Soc. Rev. 1995, 24, 391–400.
- 2
- 2aP. D. Harvey, Coord. Chem. Rev. 1996, 153, 175–198;
- 2bP. Pyykkö, Chem. Rev. 1988, 88, 563–594;
- 2cP. Pyykkö, Chem. Rev. 1997, 97, 597–636.
- 3P. Pyykkö, Angew. Chem. 2004, 116, 4512–4557;
10.1002/ange.200300624 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4412–4456, and references therein.
- 4J. M. Poblet, M. Benard, Chem. Commun. 1998, 11, 1179–1180.
- 5For example, see:
- 5aK. Jin, X. Huang, L. Pang, J. Li, A. Appel, S. Wherland, Chem. Commun. 2002, 2872–2873;
- 5bX.-C. Huang, J.-P. Zhang, X.-M. Chen, J. Am. Chem. Soc. 2004, 126, 13 218–13 219.
- 6
- 6aC.-M. Che, Z. Mao, V. M. Miskowski, M.-C. Tse, C.-K. Chan, K.-K. Cheung, D. L. Phillips, K.-H. Leung, Angew. Chem. 2000, 112, 4250–4254;
Angew. Chem. Int. Ed. 2000, 39, 4084–4088;
10.1002/1521-3773(20001117)39:22<4084::AID-ANIE4084>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 6bD. L. Phillips, C.-M. Che, K.-H. Leung, Z. Mao, M.-C. Tse, Coord. Chem. Rev. DOI: 10.1016/j.ccr.2004.09.015, and references therein.
- 7For example, see:
- 7aF. A. Cotton, X. Feng, M. Matusz, R. Poli, J. Am. Chem. Soc. 1988, 110, 7077–7083;
- 7bH. L. Hermann, G. Boche, P. Schwerdtfeger, Chem. Eur. J. 2001, 7, 5333–5342;
10.1002/1521-3765(20011217)7:24<5333::AID-CHEM5333>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 7cL. Magnko, M. Schweizer, G. Rauhut, M. Schütz, H. Stoll, H.-J. Werner, Phys. Chem. Chem. Phys. 2002, 4, 1006–1013;
- 7dE. O'Grady, N. Kaltsoyannis, Phys. Chem. Chem. Phys. 2004, 6, 680–687.
- 8M. A. Carvajal, S. Alvarez, J. J. Novoa, Chem. Eur. J. 2004, 10, 2117–2132.
- 9
- 9aK. Singh, J. R. Long, P. Stavropoulos, J. Am. Chem. Soc. 1997, 119, 2942–2943;
- 9bM. K. Ehlert, S. J. Rettig, A. Storr, R. C. Thompson, J. Trotter, Can. J. Chem. 1990, 68, 1444–1449;
- 9cH. V. R. Dias, S. A. Polach, Z. Wang, J. Fluorine Chem. 2000, 103, 163–169;
- 9dH. V. R. Dias, H. V. K. Diyabalanage, M. A. Rawashdeh-Omary, M. A. Franzman, M. A. Omary, J. Am. Chem. Soc. 2003, 125, 12 072–12 073.
- 10
- 10aX.-M. Zhang, M.-L. Tong, M.-L. Gong, H.-K. Lee, L. Luo, K.-F. Li, Y.-X. Tong, X.-M. Chen, Chem. Eur. J. 2002, 8, 3187–3194;
10.1002/1521-3765(20020715)8:14<3187::AID-CHEM3187>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 10bJ.-P. Zhang, Y.-B. Wang, X.-C. Huang, Y.-Y. Ling, X.-M. Chen, Chem. Eur. J. 2005, 11, 552–561.
- 11G. Boche, F. Bosold, M. Marsch, K. Harms, Angew. Chem. 1998, 110, 1779–1781;
10.1002/(SICI)1521-3757(19980619)110:12<1779::AID-ANGE1779>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1684–1686.10.1002/(SICI)1521-3773(19980703)37:12<1684::AID-ANIE1684>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 12G. Margraf, J. W. Bats, M. Bolte, H.-W. Lerner, M. Wagner, Chem. Commun. 2003, 956–957.
- 13A. Sundararaman, L. N. Zakharov, A. L. Rheingold, F. Jäkle, Chem. Commun. 2005, 1708–1710.
- 14R. D. Kön, G. Seifert, Z. Pan, M. F. Mahon, G. Kociok-Kön, Angew. Chem. 2003, 115, 818–821; Angew. Chem. Int. Ed. 2003, 42, 793–796.
- 15
- 15aM. A. Omary, H. H. Patterson, J. Am. Chem. Soc. 1998, 120, 7696–7705;
- 15bH. H. Patterson, S. M. Kanan, M. A. Omary, Coord. Chem. Rev. 2000, 208, 227–241, and references therein.
- 16P. Coppens, O. Gerlits, I. I. Vorontsov, A. Yu. Kovalevsky, Y.-S. Chen, T. Graber, I. V. Novozhilova, Chem. Commun. 2004, 2144–2145; P. Coppens, I. I. Vorontsov, T. Graber, M. Gembicky, A. Yu. Kovalevsky, Acta Crystallogr. A 2005, 61, 162–172, and references therein.
- 17P. Coppens, I. I. Vorontsov, A. Yu. Kovalevsky, Y.-S. Chen, T. Graber, I. V. Novozhilova, Phys. Rev. Lett. 2005, 94, 193 003.
- 18
- 18aH. V. R. Dias, H. V. K. Diyabalanage, M. A. Rawashdeh-Omary, M. A. Franzman, M. A. Omary, J. Am. Chem. Soc. 2003, 125, 12 072–12 073;
- 18bH. V. R. Dias, H. V. K. Diyabalanage, M. G. Eldabaja, O. Elbjeirami, M. A. Rawashdeh-Omary, M. A. Omary, J. Am. Chem. Soc. 2005, 127, 7489–7501.
- 19P. Coppens, B.-Q. Ma, O. Gerlits, Y. Zhang, P. Kulshrestha, CrystEngComm 2002, 4, 302–309.
- 20S.-L. Zheng, P. Coppens, Chem. Eur. J. 2005, 11, 3583–3590.
- 21N. N. Barashkov, T. V. Sakhno, R. N. Nurmukhanmetov, O. V. Khakel', Uspekhi Khimii 1993, 62, 579–593; Russ. Chem. Rev. 1993, 62, 539–552, and references therein.
- 22S.-L. Zheng, M. Gembecky, I. Vorontsov, T. Graber, M. Messerschmidt, P. Dominiak, P. Coppens, unpublished results.
- 23S.-L. Zheng, M.-L. Tong, X.-M. Chen, S. W. Ng, J. Chem. Soc. Dalton Trans. 2002, 360–364.
- 24S.-L. Zheng, P. Coppens, Cryst. Growth Des., submitted.
- 25Crystal data for 1: triclinic, space group P
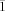 , a=6.8426(3), b=12.0173(5), c=23.859(1) Å, α=78.507(1), β=89.118(1), χ=79.825(1)°, U=1892.0(1) Å3, Z=2, ρcalcd=1.517 g cm−3, MoKα=0.71073 Å, R1=0.0437, wR2=0.1262, GOF=1.050 (see the Supporting Information).
, a=6.8426(3), b=12.0173(5), c=23.859(1) Å, α=78.507(1), β=89.118(1), χ=79.825(1)°, U=1892.0(1) Å3, Z=2, ρcalcd=1.517 g cm−3, MoKα=0.71073 Å, R1=0.0437, wR2=0.1262, GOF=1.050 (see the Supporting Information).
- 26A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Untrecht University, Utrecht, The Netherlands, 2003.
- 27
- 27aI. C. Hayes, A. J. Stone, Mol. Phys. 1984, 53, 83–105;
- 27bA. J. Stone, The Theory of Intermolecular Forces, Clarendon, Oxford, 1996.
10.1093/oso/9780198558842.001.0001 Google Scholar
- 28Similar values of 305.7 and 242.1 kJ mol−1 are obtained with MP2 BSSE correlation calculations in GAUSSIAN03 and with DFT calculations in Amsterdam Density Functional Program (ADF), respectively.
- 29
- 29aI. Mayer, Chem. Phys. Lett. 1983, 97, 270–274;
- 29bI. Mayer, Int. J. Quantum Chem. 1984, 26, 151–154;
- 29cA. J. Bridgeman, G. Cavigliasso, L. R. Ireland, J. Rothery, J. Chem. Soc. Dalton Trans. 2001, 2095–2108.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.