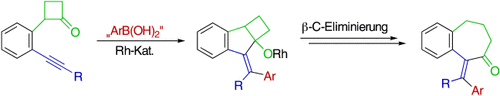
Synthesis of Seven-Membered-Ring Ketones by Arylative Ring Expansion of Alkyne-Substituted Cyclobutanones†
Takanori Matsuda Dr.
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorMasaomi Makino
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorMasahiro Murakami Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorTakanori Matsuda Dr.
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorMasaomi Makino
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorMasahiro Murakami Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615–8510, Japan, Fax: (+81) 75-383-2748
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Young Scientists (B; No. 15750085) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z500799_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. A. Petasis, M. A. Patane, Tetrahedron 1992, 48, 5757–5821;
- 1bC. J. Roxburgh, Tetrahedron 1993, 49, 10 749–10 784;
- 1cG. Mehta, V. Singh, Chem. Rev. 1999, 99, 881–930;
- 1dL. Yet, Chem. Rev. 2000, 100, 2963–3007.
- 2
- 2aG. Illuminati, L. Mandolini, Acc. Chem. Res. 1981, 14, 95–102;
- 2bC. Galli, L. Mandolini, Eur. J. Org. Chem. 2000, 3117–3125.
- 3
- 3aT. Matsuda, M. Makino, M. Murakami, Org. Lett. 2004, 6, 1257–1259;
- 3bT. Matsuda, M. Makino, M. Murakami, Bull. Chem. Soc. Jpn. 2005, in press.
- 4For intramolecular additions of an organorhodium(I) to a carbonyl group, see:
- 4aA. Takezawa, K. Yamaguchi, T. Ohmura, Y. Yamamoto, N. Miyaura, Synlett 2002, 1733–1735;
- 4bR. Shintani, K. Okamoto, Y. Otomaru, K. Ueyama, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 54–55;
- 4cT. Miura, T. Sasaki, H. Nakazawa, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1390–1391;
- 4dT. Miura, M. Shimada, M. Murakami, Synlett 2005, 667–669; For intermolecular additions, see:
- 4eM. Sakai, M. Ueda, M. Miyaura, Angew. Chem. 1998, 110, 3475–3477;
10.1002/(SICI)1521-3757(19981204)110:23<3475::AID-ANGE3475>3.0.CO;2-W Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 3279–3281;10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 4fC. Krug, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 1674–1679;
- 4gM. Pucheault, S. Darses, J.-P. Genet, J. Am. Chem. Soc. 2004, 126, 15 356–15 357.
- 5Wittig olefination of the aldehyde with cyclopropylidenephosphorane afforded 2-bromobenzylidenecyclopropane (71 %). Oxidation of the methylenecyclopropane with m-chloroperbenzoic acid followed by treatment with aqueous HBF4 gave 2-(2-bromophenyl)cyclobutanone (58 %). Introduction of a but-1-ynyl moiety by palladium-catalyzed coupling furnished 1 a (55 %). See Supporting Information for details.
- 6Triphenylboroxin and water were used to generate phenylboronic acid in situ, see:
- 6aT. Senda, M. Ogasawara, T. Hayashi, J. Org. Chem. 2001, 66, 6852–6856;
- 6bT. Hayashi, K. Inoue, N. Taniguchi, M. Ogasawara, J. Am. Chem. Soc. 2001, 123, 9918–9919.
- 7
- 7aM. Murakami, H. Igawa, Helv. Chim. Acta 2002, 85, 4182–4188;
- 7bM. Lautens, M. Yoshida, Org. Lett. 2002, 4, 123–125;
- 7cM. Lautens, T. Marquardt, J. Org. Chem. 2004, 69, 4607–4614;
- 7dT. Miura, M. Shimada, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1094–1095.
- 8For a review on β-carbon eliminations from palladium(II) cyclobutanolates, see: T. Nishimura, S. Uemura, Synlett 2004, 201–216.
- 9For examples of related migrations of transition metals leading to enolates, see:
- 9aH. Qian, R. A. Widenhoefer, J. Am. Chem. Soc. 2003, 125, 2056–2057;
- 9bS. V. Gagnier, R. C. Larock, J. Am. Chem. Soc. 2003, 125, 4804–4807.
- 10
- 10aK. Oguma, M. Miura, T. Satoh, M. Nomura, J. Am. Chem. Soc. 2000, 122, 10 464–10 465;
- 10bR. Shintani, K. Okamoto, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 2872–2873;
- 10cH. Yamabe, A. Mizuno, H. Kusama, N. Iwasawa, J. Am. Chem. Soc. 2005, 127, 3248–3249.
- 11The reaction with o-tolylboroxin resulted in the formation of a complex mixture of products, in which 1,2-adduct 4 was the only identifiable product.
- 12Ring expansion by β-carbon elimination failed to occur even when the isolated 5 ja was heated at 160 °C in the presence of the rhodium catalyst.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.