Tungsten–Lead Triple Bonds: Syntheses, Structures, and Coordination Chemistry of the Plumbylidyne Complexes trans-[X(PMe3)4WPb(2,6-Trip2C6H3)]†
Alexander C. Filippou Prof. Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorNils Weidemann Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorGregor Schnakenburg Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorHolger Rohde Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorAthanassios I. Philippopoulos Dr.
Institute of Physical Chemistry, NCSR Demokritos, Ag. Paraskevi Attikis, 15310 Athens, Greece
Search for more papers by this authorAlexander C. Filippou Prof. Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorNils Weidemann Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorGregor Schnakenburg Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorHolger Rohde Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor Strasse 2, 12489 Berlin, Germany, Fax: (+49) 30-2093-6939
Search for more papers by this authorAthanassios I. Philippopoulos Dr.
Institute of Physical Chemistry, NCSR Demokritos, Ag. Paraskevi Attikis, 15310 Athens, Greece
Search for more papers by this authorWe are grateful to the Humboldt-Universität zu Berlin and the Deutsche Forschungsgemeinschaft (project FI 445/6-1) for the generous financial support of this work, Dr. B. Ziemer and P. Neubauer for the X-ray diffraction studies, Dr. U. Hartmann and U. Kätel for the elemental analyses, and W.-D. Bloedorn and A. Thiesies for the 2D NMR spectra. N. Weidemann thanks the Fonds der Chemischen Industrie for a fellowship. X=Br, I; Trip=2,4,6-iPr3C6H2.
Graphical Abstract
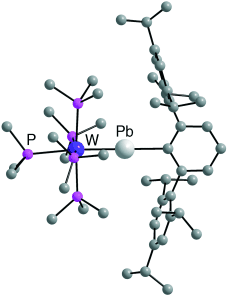
Senkblei: Die vorgestellten Verbindungen trans-[L(PMe3)4WPb(2,6-Trip2C6H3)]n+ (n=0: L=Br, I; n=1: L=PhCN, PMe3; Trip=2,4,6-iPr3C6H2; die Struktur des Derivats mit L=PMe3 ist gezeigt) enthalten alle eine W-Pb-Dreifachbindung. Quantenchemische Analysen der WPb-Bindung im Plumbylidinkomplex [(PMe3)5WPb(2,6-Trip2C6H3)]+ bestätigen die elektronische Analogie zu Carbinkomplexen vom Fischer-Typ.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461725_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Review articles on multiple bonds of the heavier group homologues of carbon:
- 1aG. Raabe, J. Michl, Chem. Rev. 1985, 85, 419;
- 1bA. G. Brook, K. M. Baines, Adv. Organomet. Chem. 1986, 25, 1;
- 1cR. West, Angew. Chem. 1987, 99, 1231; Angew. Chem. Int. Ed. Engl. 1987, 26, 1201;
- 1dJ. Barrau, J. Escudié, J. Satgé, Chem. Rev. 1990, 90, 283;
- 1eT. Tsumuraya, S. A. Batcheller, S. Masamune, Angew. Chem. 1991, 103, 916; Angew. Chem. Int. Ed. Engl. 1991, 30, 902;
- 1fR. S. Grev, Adv. Organomet. Chem. 1991, 33, 125;
- 1gM. Weidenbruch, Coord. Chem. Rev. 1994, 130, 275;
- 1hJ. Escudié, C. Couret, H. Ranaivonjatovo, J. Satgé, Coord. Chem. Rev. 1994, 130, 427;
- 1iR. Okazaki, R. West, Adv. Organomet. Chem. 1996, 39, 231;
- 1jM. Driess, H. Grützmacher, Angew. Chem. 1996, 108, 900; Angew. Chem. Int. Ed. Engl. 1996, 35, 828;
- 1kK. M. Baines, W. G. Stibbs, Adv. Organomet. Chem. 1996, 39, 275;
- 1lJ. Escudié, C. Couret, H. Ranaivonjatovo, Coord. Chem. Rev. 1998, 178, 565;
- 1mJ. Escudié, H. Ranaivonjatovo, Adv. Organomet. Chem. 1999, 44, 113;
- 1nM. Weidenbruch, Eur. J. Inorg. Chem. 1999, 373;
10.1002/(SICI)1099-0682(199903)1999:3<373::AID-EJIC373>3.0.CO;2-D CAS Web of Science® Google Scholar
- 1oP. P. Power, Chem. Rev. 1999, 99, 3463;
- 1pM. Weidenbruch, J. Organomet. Chem. 2002, 646, 39.
- 2The name diplumbene underlines the similar composition of [Pb2R4] and alkenes, and is used herein instead of the term bis(plumbanediyl) which is more appropriate to describe the physical properties, structures, and chemical behavior of these compounds, which bear little resemblance to those of alkenes.
- 3
- 3aK. W. Klinkhammer, T. F. Fässler, H. Grützmacher, Angew. Chem. 1998, 110, 114;
10.1002/(SICI)1521-3757(19980116)110:1/2<114::AID-ANGE114>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 1998, 37, 124;10.1002/(SICI)1521-3773(19980202)37:1/2<124::AID-ANIE124>3.0.CO;2-C CAS Web of Science® Google Scholar
- 3bM. Stürmann, M. Weidenbruch, K. W. Klinkhammer, F. Lissner, H. Marsmann, Organometallics 1998, 17, 4425;
- 3cM. Stürmann, W. Saak, H. Marsmann, M. Weidenbruch, Angew. Chem. 1999, 111, 145;
10.1002/(SICI)1521-3757(19990115)111:1/2<145::AID-ANGE145>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 187;10.1002/(SICI)1521-3773(19990115)38:1/2<187::AID-ANIE187>3.0.CO;2-2 CAS Web of Science® Google Scholar
- 3dM. Stürmann, W. Saak, M. Weidenbruch, K. W. Klinkhammer, Eur. J. Inorg. Chem. 1999, 579.
- 4A PbPb multiple bond has been also formulated in the complex anion [{W(CO)5}4Pb2]2−: P. Rutsch, G. Huttner, Angew. Chem. 2000, 112, 3852;
10.1002/1521-3757(20001016)112:20<3852::AID-ANGE3852>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3697.10.1002/1521-3773(20001016)39:20<3697::AID-ANIE3697>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 5
- 5aJ. D. Cotton, P. J. Davidson, M. F. Lappert, J. Chem. Soc. Dalton Trans. 1976, 2275;
- 5bM. F. Lappert, P. P. Power, J. Chem. Soc. Dalton Trans. 1985, 51;
- 5cH.-J. Kneuper, E. Herdtweck, W. A. Herrmann, J. Am. Chem. Soc. 1987, 109, 2508;
- 5dM. F. Lappert, R. S. Rowe, Coord. Chem. Rev. 1990, 100, 267.
- 6Selected recent review articles on carbene complexes:
- 6aM. A. Sierra, Chem. Rev. 2000, 100, 3591;
- 6bA. de Meijere, H. Schirmer, M. Duetsch, Angew. Chem. 2000, 112, 4124;
10.1002/1521-3757(20001117)112:22<4124::AID-ANGE4124>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3964;10.1002/1521-3773(20001117)39:22<3964::AID-ANIE3964>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 6cT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18;
- 6dA. H. Hoveyda, R. R. Schrock, Chem. Eur. J. 2001, 7, 945;
10.1002/1521-3765(20010302)7:5<945::AID-CHEM945>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 6eW. A. Herrmann, Angew. Chem. 2002, 114, 1342;
Angew. Chem. Int. Ed. 2002, 41, 1290;
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 6fJ. W. Herndon, Coord. Chem. Rev. 2003, 243, 3;
- 6gW. Kirmse, Angew. Chem. 2003, 115, 1120;
10.1002/ange.200390261 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1088.
- 7
- 7aW. A. Herrmann, H.-J. Kneuper, E. Herdtweck, Angew. Chem. 1985, 97, 1060; Angew. Chem. Int. Ed. Engl. 1985, 24, 1062;
- 7bF. Ettel, G. Huttner, L. Zsolnai, Angew. Chem. 1989, 101, 1525; Angew. Chem. Int. Ed. Engl. 1989, 28, 1496;
- 7cF. Ettel, M. Schollenberger, B. Schiemenz, G. Huttner, L. Zsolnai, J. Organomet. Chem. 1994, 476, 153.
- 8
- 8aM. J. S. Dewar, M. K. Holloway, G. L. Grady, J. J. P. Stewart, Organometallics 1985, 4, 1973;
- 8bG. Trinquier, J. Am. Chem. Soc. 1990, 112, 2130;
- 8cH. Jacobsen, T. Ziegler, J. Am. Chem. Soc. 1994, 116, 3667.
- 9W. Kutzelnigg, Angew. Chem. 1984, 96, 262; Angew. Chem. Int. Ed. Engl. 1984, 23, 272.
- 10For the relativistic contraction of the 6s orbital of lead and the increase of the 6s–6p orbital energy gap causing the inert-pair effect see:
- 10aP. Pyykkö, Chem. Rev. 1988, 88, 563;
- 10bN. Kaltsoyannis, J. Chem. Soc. Dalton Trans. 1997, 1.
- 11A. C. Filippou, H. Rohde, G. Schnakenburg, Angew. Chem. 2004, 116, 2293;
10.1002/ange.200353477 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2243.
- 12
- 12aE. O. Fischer, Adv. Organomet. Chem. 1976, 14, 1;
- 12bH. Fischer, P. Hofmann, F. R. Kreißl, R. R. Schrock, K. Weiss, Carbyne Complexes, VCH, Weinheim, 1988;
- 12cA. Mayr, H. Hoffmeister, Adv. Organomet. Chem. 1991, 32, 227.
- 13E. Carmona, A. Galindo, M. L. Poveda, R. D. Rogers, Inorg. Chem. 1985, 24, 4033.
- 14L. Pu, B. Twamley, P. P. Power, Organometallics 2000, 19, 2874.
- 15The Supporting Information contains the experimental section including the syntheses, spectroscopic, and crystallographic data of 1-I and of the plumbylidyne complexes 2-Br, 2-I, 3, and 4. It also contains IR and NMR spectroscopic data of the dinitrogen complexes cis-[W(N2)2(PMe3)4] and [W(N2)(PMe3)5] and details of the electronic structure calculations of the complex cation [(PMe3)5WPb(2,6-Trip2C6H3)]+. CCDC-247928 (1-I⋅(n-C5H12)), CCDC-247927 (2-Br), CCDC-247929 (2-I⋅0.5 (n-C5H12)), CCDC-247931 (3) and CCDC-247930 (4⋅0.705 (C6H5F)) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 16The only other known organolead(II) iodide is [Pb(η-C5H5)I]: A. K. Holliday, P. H. Makin, R. J. Puddephatt, J. Chem. Soc. Dalton Trans. 1976, 435.
- 17The Pb⋅⋅⋅Pb separation of 1-I is much longer than the interatomic distance in elementary lead (3.494 Å):
- 17aH. P. Klug, J. Am. Chem. Soc. 1946, 68, 1493;
- 17bA. F. Wells, Structural Inorganic Chemistry, 5th ed., Clarendon, Oxford, 1984, p. 1288.
- 18IR spectra of the reaction solutions and the 1H and 31P{1H} NMR spectra of the crude products, that were isolated after completion of the reaction, revealed the concomitant formation of two by-products. These were identified to be [W(N2)(PMe3)5] and 1,3-Trip2C6H4 by comparison with authentic samples (Supporting Information). A gray-green solid, which is soluble in THF, but insoluble in pentane was also formed in these reactions. This solid burned upon exposure to air in dried form and was not further characterized.
- 19The term “electron-rich” is used herein to emphasize the strong π-electron donating ability of the metal centers in these complexes:
- 19aA. J. L. Pombeiro, R. L. Richards, Coord. Chem. Rev. 1990, 104, 13;
- 19bA. J. L. Pombeiro, M. F. C. Guedes da Silva, R. A. Michelin, Coord. Chem. Rev. 2001, 218, 43.
- 20The orientation of the para-positioned isopropyl substituents lowers the molecular symmetry of 2-Br and 2-I from roughly C2v to C2. The terphenyl substituent adopts in both plumbylidyne complexes an eclipsed conformation, as shown by the interplane angle of 5.9(2) (2-Br) and 9.0(1)° (2-I) between the central aryl ring and the least-square plane through the atoms Br (I), W, P1, and P3.
- 21Only a few compounds with WPb bonds have been structurally characterized. The WPb bond lengths range in these compounds from 2.7423(3) to 3.339(1) Å depending on the bond order, the oxidation states, and the coordination numbers of lead and tungsten:
- 21aref. [4];
- 21bref. [14];
- 21cS. Seebald, G. Kickelbick, F. Möller, U. Schubert, Chem. Ber. 1996, 129, 1131;
- 21dL. Pu, P. P. Power, I. Boltes, R. Herbst-Irmer, Organometallics 2000, 19, 352;
- 21eN. Seidel, K. Jacob, A. K. Fischer, Organometallics 2001, 20, 578;
- 21fJ. Campbell, H. P. A. Mercier, H. Franke, D. P. Santry, D. A. Dixon, G. J. Schrobilgen, Inorg. Chem. 2002, 41, 86.
- 22L. M. Atagi, J. M. Mayer, Polyhedron 1995, 14, 113.
- 23A. C. Filippou, N. Weidemann, H. Rohde, unpublished results.
- 24No 207Pb satellites were observed in the 31P{1H} NMR spectra of 2-Br, 2-I, 3, and 4 at room temperature, and attempts to detect the 207Pb NMR signal of 2-Br in C6D6 at ambient temperature were not successful to date.
- 25
- 25aA. C. Filippou, E. O. Fischer, J. Organomet. Chem. 1990, 383, 179;
- 25bA. C. Filippou, C. Mehnert, K. M. A. Wanninger, M. Kleine, J. Organomet. Chem. 1995, 491, 47;
- 25cA. C. Filippou, D. Wössner, G. Kociok-Köhn, I. Hinz, L. Grubert, J. Organomet. Chem. 1997, 532, 207;
- 25dF. W. Lee, M. C. W. Chan, K. K. Cheung, C. M. Che, J. Organomet. Chem. 1998, 563, 191;
- 25eE. Bannwart, H. Jacobsen, R. Hübener, H. W. Schmalle, H. Berke, J. Organomet. Chem. 2001, 622, 97.
- 26
- 26aS. R. Bahr, P. Boudjouk, J. Org. Chem. 1992, 57, 5545;
- 26bR. Taube, S. Wache, J. Organomet. Chem. 1992, 428, 431.
- 27
- 27aA. G. Massey, A. J. Park, J. Organomet. Chem. 1964, 2, 245;
- 27bJ. B. Lambert, S. Zhang, S. M. Ciro, Organometallics 1994, 13, 2430.
- 28For the trans-influence of carbyne and germylidyne ligands see:
- 28aref. [12b];
- 28bE. Bannwart, H. Jacobsen, F. Furno, H. Berke, Organometallics 2000, 19, 3605;
- 28cF. Furno, T. Fox, H. W. Schmalle, H. Berke, J. Organometallics 2000, 19, 3620;
- 28dA. C. Filippou, P. Portius, A. I. Philippopoulos, Organometallics 2002, 21, 653.
- 29For d6 tungsten nitrile complexes revealing dπ(tungsten)→π*(nitrile) back-bonding see:
- 29aJ. Chatt, G. J. Leigh, H. Neukomm, C. J. Pickett, D. R. Stanley, J. Chem. Soc. Dalton Trans. 1980, 121;
- 29bB. J. Carter, J. E. Bercaw, H. B. Gray, J. Organomet. Chem. 1979, 181, 105;
- 29cH. Seino, Y. Tanabe, Y. Ishii, M. Hidai, Inorg. Chim. Acta 1998, 280, 163;
- 29dC. M. Habeck, N. Lehnert, C. Näther, F. Tuczek, Inorg. Chim. Acta 2002, 337, 11.
- 30
- 30aU. Schubert, E. O. Fischer, D. Wittmann, Angew. Chem. 1980, 92, 662; Angew. Chem. Int. Ed. Engl. 1980, 19, 643;
- 30bU. Schubert, D. Neugebauer, P. Hofmann, B. E. R. Schilling, H. Fischer, A. Motsch, Chem. Ber. 1981, 114, 3349;
- 30cE. O. Fischer, D. Wittmann, D. Himmelreich, U. Schubert, K. Ackermann, Chem. Ber. 1982, 115, 3141;
- 30dE. O. Fischer, D. Wittmann, D. Himmelreich, R. Cai, K. Ackermann, D. Neugebauer, Chem. Ber. 1982, 115, 3152.
- 31A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. 1988, 88, 899.
- 32The ΔEorb term could not be broken down into the σ and π bond energy contributions owing to the lack of symmetry. The percentage contribution of ΔEorb to the total attractive interactions (ΔEorb and ΔEelstat) reflects the covalent character of the bond.
- 33G. Frenking, N. Fröhlich, Chem. Rev. 2000, 100, 717 and references therein.
- 34[W(PMe3)5] has a singlet ground-state configuration and adopts a distorted trigonal-bipyramidal minimum geometry, in which one of the equatorial PMe3 ligands displays a CH agostic interaction with the metal center (see Supporting Information). [W(PMe3)5] has been suggested as an intermediate in the cyclometallation reaction of [W(PMe3)6] to afford [W(PMe3)4(η2-CH2PMe2)H] and PMe3: D. Rabinovich, G. Parkin, J. Am. Chem. Soc. 1990, 112, 5381.
- 35The ion [Pb(2,6-Trip2C6H3)]+ has a singlet ground state (see Supporting Information). The toluene adduct of [Pb(2,6-Trip2C6H3)]+ was isolated recently with the counterion [B(Me)(C6F5)3]−: S. Hino, M. Brynda, A. D. Phillips, P. P. Power, Angew. Chem. 2004, 116, 2709;
10.1002/ange.200353365 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2655.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.