Synthesis of the C-1027 Chromophore Framework through Atropselective Macrolactonization†
Masayuki Inoue Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan
Search for more papers by this authorTakeo Sasaki Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorSuguru Hatano
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorMasahiro Hirama Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorMasayuki Inoue Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan
Search for more papers by this authorTakeo Sasaki Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorSuguru Hatano
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorMasahiro Hirama Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorThis work was supported by CREST, Japan Science and Technology Agency (JST). A fellowship to T.S. from the Japanese Society for the Promotion of Science (JSPS) is gratefully acknowledged.
Graphical Abstract
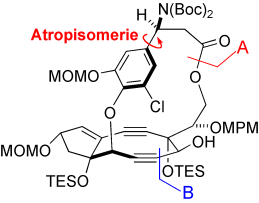
Weit reichende sterische Wechselwirkungen zwischen den 1,5-Diol-Schutzgruppen wurden bei der Synthese des Chromophorgerüsts von C-1027 für die erfolgreiche atropselektive Makrolactonisierung (A im Schema) genutzt. Darauf folgende LiN(SiMe3)2/CeCl3-unterstützte Acetylidaddition an eine Aldehydeinheit im System des starren Makrocyclus (B) lieferte das neungliedrige Zielringsystem mit einer hochgespannten Diineinheit.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461428_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Otani, Y. Minami, T. Marunaka, R. Zhang, M.-Y. Xie, J. Antibiot. 1988, 41, 1580–1585;
- 1bY. Zhen, S. Ming, Y. Bin, T. Otani, H. Saito, Y. Yamada, J. Antibiot. 1989, 42, 1294–1298.
- 2 Neocarzinostatin (Eds.: ), Springer, Tokyo, 1997.
- 3
- 3aJ. E. Leet, D. R. Schroeder, S. J. Hofstead, J. Golik, K. L. Colson, S. Huang, S. E. Klohr, T. W. Doyle, J. A. Matson, J. Am. Chem. Soc. 1992, 114, 7946–7948;
- 3bS. Kawata, S. Ashizawa, M. Hirama, J. Am. Chem. Soc. 1997, 119, 12 012–12 013.
- 4D. R. Schroeder, K. L. Colson, S. E. Klohr, N. Zein, D. R. Langley, M. S. Lee, J. A. Matson, T. W. Doyle, J. Am. Chem. Soc. 1994, 116, 9351–9352.
- 5For a review on chromoprotein antibiotics and other enediyne natural products, see: Z. Xi, I. H. Goldberg in Comprehensive Natural Products Chemistry, Vol. 7 (Eds.: ), Elsevier, Dordrecht, 1999, pp. 553–592.
10.1016/B978-0-08-091283-7.00070-9 Google Scholar
- 6
- 6aY. Minami, K. Yoshida, R. Azuma, M. Saeki, T. Otani, Tetrahedron Lett. 1993, 34, 2633–2636;
- 6bK. Yoshida, Y. Minami, R. Azuma, M. Saeki, T. Otani, Tetrahedron Lett. 1993, 34, 2637–2640;
- 6cK. Yoshida, Y. Minami, T. Otani, Y. Tada, M. Hirama, Tetrahedron Lett. 1994, 35, 5253–5256;
- 6dK. Iida, S. Fukuda, T. Tanaka, M. Hirama, S. Imajo, M. Ishiguro, K. Yoshida, T. Otani, Tetrahedron Lett. 1996, 37, 4997–5000.
- 7
- 7aY. Sugiura, T. Matsumoto, Biochemistry 1993, 32, 5548–5553;
- 7bY. Xu, X. Zhen, Y. Zhen. I. H. Goldberg, Biochemistry 1995, 34, 12 451–12 460.
- 8Total syntheses of the nine-membered ring enediynes were reported: N-1999A2:
- 8aS. Kobayashi, S. Ashizawa, Y. Takahashi, Y. Sugiura, M. Nagaoka, M. J. Lear, M. Hirama, J. Am. Chem. Soc. 2001, 123, 11 294–11 295; neocarzinostatin chromophore:
- 8bA. G. Myers, J. Liang, M. Hammond, P. M. Harrington, Y. Wu, E, Y. Kuo, J. Am. Chem. Soc. 1998, 120, 5319–5320;
- 8cA. G. Myers, R. Glatthar, M. Hammond, P. M. Harrington, E, Y. Kuo, J. Liang, S. E. Schaus, Y. Wu, J.-N. Xiang, J. Am. Chem. Soc. 2002, 124, 5380–5401.
- 9For recent synthetic studies of the enediyne natural products from this laboratory, see: neocarzinostatin chromophore:
- 9aK. Toyama, S. Iguchi, H. Sakazaki, T. Oishi, M. Hirama, Bull. Chem. Soc. Jpn. 2001, 74, 997–1008; kedarcidin:
- 9bF. Yoshimura, S. Kawata, M. Hirama, Tetrahedron Lett. 1999, 40, 8281–8285;
- 9cI. Ohashi, M. J. Lear, F. Yoshimura, M. Hirama, Org. Lett. 2004, 6, 719–722; maduropeptin:
- 9dN. Kato, S. Shimamura, Y. Kikai, M. Hirama, Synlett 2004, 2107–2110, and references therein.
- 10For the synthesis of the protected kedarcidin chromophore aglycon, see: A. G. Myers, P. C. Hogan, A. R. Hurd, S. D. Goldberg, Angew. Chem. 2002, 114, 1104–1109;
10.1002/1521-3757(20020315)114:6<1104::AID-ANGE1104>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1062–1066.10.1002/1521-3773(20020315)41:6<1062::AID-ANIE1062>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 11For reviews on the syntheses of enediyne compounds, see:
- 11aK. C. Nicolaou, W.-M. Dai, Angew. Chem. 1991, 103, 1453–1481; Angew. Chem. Int. Ed. Engl. 1991, 30, 1387–1416;
- 11bS. J. Danishefsky, M. D. Shair, J. Org. Chem. 1996, 61, 16–44.
- 12M. Shibuya, H. Sakurai, T. Maeda, E. Nishiwaki, M. Saito, Tetrahedron Lett. 1986, 27, 1351–1354.
- 13
- 13aK. Iida, T. Ishii, M. Hirama, T. Otani, Y. Minami, K. Yoshida, Tetrahedron Lett. 1993, 34, 4079–4082;
- 13bM. F. Semmelhack, Y. Jiang, D. Ho, Org. Lett. 2001, 3, 2403–2406.
- 14For a review, see: P. Lloyd-Williams, E. Giralt, Chem. Soc. Rev. 2001, 30, 145–157.
- 15Only a few examples have been reported on the stereoselective synthesis of nonbiaryl stereogenic axes in natural product syntheses; see:
- 15aD. A. Evans, M. R. Wood, B. W. Trotter, T. I. Richardson, J. C. Barrow, J. L. Katz, Angew. Chem. 1998, 110, 2864–2868;
10.1002/(SICI)1521-3757(19981002)110:19<2864::AID-ANGE2864>3.0.CO;2-R Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2700–2703;10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P CAS Web of Science® Google Scholar
- 15bD. A. Evans, C. J. Dinsmore, P. S. Watson, M. R. Wood, T. I. Richardson, B. W. Trotter, J. L. Katz, Angew. Chem. 1998, 110, 2868–2872;
10.1002/(SICI)1521-3757(19981002)110:19<2868::AID-ANGE2868>3.0.CO;2-3 Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2704–2708;10.1002/(SICI)1521-3773(19981016)37:19<2704::AID-ANIE2704>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 15cM. E. Layton, C. A. Morales, M. D. Shair, J. Am. Chem. Soc. 2002, 124, 773–775;
- 15dK. C. Nicolaou, C. N. C. Boddy, J. Am. Chem. Soc. 2002, 124, 10 451–10 455; see also references [9b] and [10].
- 16I. Sato, Y. Akahori, T. Sasaki, T. Kikuchi, M. Hirama, Chem. Lett. 1999, 867–868.
- 17M. Inoue, S. Hatano, M. Kodama, T. Sasaki, T. Kikuchi, M. Hirama, Org. Lett. 2004, 6, 3833–3836.
- 18K. Iida, M. Hirama, J. Am. Chem. Soc. 1994, 116, 10 310–10 311.
- 19
- 19aT. Sasaki, M. Inoue, M. Hirama, Tetrahedron Lett. 2001, 42, 5299–5303;
- 19bM. Inoue, T. Kikuchi, M. Hirama, Tetrahedron Lett. 2004, 45, 6439–6442.
- 20For other studies on bicyclo[7.3.0]dodecadiyne formation, see:
- 20aP. A. Wender, M. Harmata, D. Jeffery, C. Mukai, J. Suffert, Tetrahedron Lett. 1988, 29, 909–912;
- 20bP. A. Wender, J. A. McKinney, C. Mukai, J. Am. Chem. Soc. 1990, 112, 5369–5370;
- 20cT. Doi, T. Takahashi, J. Org. Chem. 1991, 56, 3465–3467;
- 20dP. Magnus, R. Carter, M. Davies, J. Elliott, T. Pitterna, Tetrahedron 1996, 52, 6283–6306;
- 20eH. Tanaka, H. Yamada, A. Matsuda, T. Takahashi, Synlett 1997, 381–383;
- 20fS. Caddick, V. M. Delisser, V. E. Doyle, S. Khan, A. G. Avent, S. Vile, Tetrahedron 1999, 55, 2737–2754, and references therein.
- 21I. Sato, T. Kikuchi, M. Hirama, Chem. Lett. 1999, 511–512.
- 22
- 22aM. Hirama, T. Gomibuchi, K. Fujiwara, Y. Sugiura, M. Uesugi, J. Am. Chem. Soc. 1991, 113, 9851–9853;
- 22bC. R. Johnson, M. P. Braun, J. Am. Chem. Soc. 1993, 115, 11 014–11 015.
- 23S. Kawata, M. Hirama, Tetrahedron Lett. 1998, 39, 8707–8710.
- 24K. Sonogashira in Comprehensive Organic Synthesis, Vol. 3 (Eds.: ), Pergamon, London, 1990, pp. 521–549.
- 25E. J. Corey, K. C. Nicolaou, J. Am. Chem. Soc. 1974, 96, 5614–5616.
- 26
- 26aI. Shiina, M. Kubota, R. Ibuka, Tetrahedron Lett. 2002, 43, 7535–7539;
- 26bI. Shiina, M. Kubota, H. Oshiumi, M. Hashizume, J. Org. Chem. 2004, 69, 1822–1830.
- 27For an excellent example of the selective isomerization of atropisomers in the context of a total synthesis of vancomycin, see: D. L. Boger, S. Miyazaki, S. H. Kim, J. H. Wu, S. L. Castle, O. Loiseleur, Q. Jin, J. Am. Chem. Soc. 1999, 121, 10 004–10 011.
- 28Interestingly, the facile atropisomerism of kedarcidin synthetic intermediates has been observed: A. G. Myers, A. R. Hurd, P. C. Hogan, J. Am. Chem. Soc. 2002, 124, 4583–4585. see also reference [10].
- 29F. Mohamadi, N. G. J. Richards, W. C. Guida, R. Liskamp, M. Lipton, C. Caufield, G. Chang, T. Hendrickson, W. C. Still, J. Comput. Chem. 1990, 11, 440–467.
- 30J. L. Halcombe, T. Livinghouse, J. Org. Chem. 1986, 51, 111–114.
- 31Yamaguchi lactonization of 31 gave 32 in lower yield (32: 33 %, 33: 6 %, dimer: 30 %): J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993.
- 32Bis-Boc protection of the amine was found to have a dramatic effect on the yield of the nine-membered ring cyclization.[19 b]
- 33Cope rearrangement of the nine-membered cyclic 1,5-diyne to the bisallene is considered to be the major decomposition pathway.[18]
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.