A Catalytic Carbon–Phosphorus Ylide Reaction: Phosphane-Catalyzed Annulation of Allylic Compounds with Electron-Deficient Alkenes†
Yishu Du Dr.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorXiyan Lu Prof.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorChunming Zhang Dr.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorYishu Du Dr.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorXiyan Lu Prof.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorChunming Zhang Dr.
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China, Fax: (+86) 21-6416-6128
Search for more papers by this authorThis work was funded by the Major State Basic Research Program (grant G2000077502-A). We also thank the National Natural Science Foundation of China and the Chinese Academy of Sciences for financial support.
Graphical Abstract
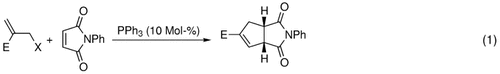
Leicht verfügbare Edukte, hohe Selektivität – dies sind die attraktiven Merkmale der Titelreaktion. Bromid-, Acetat- und tert-Butylcarbonat-Derivate von durch eine Morita-Baylis-Hillman-Reaktion erhaltenen Addukten reagieren mit PPh3 zu Allylyliden. Die phosphankatalysierte Anellierung der Ylide mit elektronenarmen Alkenen ergibt stereoselektiv funktionalisierte Cyclopentene [Gl. (1)]. X=Br, OAc, OBoc; E=CO2Et.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z50449_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aO. I. Kolodiazhnyi, Phosphorus Ylides: Chemistry and Application in Organic Synthesis, Wiley-VCH, New York, 1999;
10.1002/9783527613908 Google Scholar
- 1bB. E. Maryanoff, A. B. Reitz, Chem. Rev. 1989, 89, 863, and references therein.
- 2A.-H. Li, L.-X. Dai, V. K. Aggarwal, Chem. Rev. 1997, 97, 2341.
- 3For a catalytic P–Te ylide reaction, see: L.-B. Han, F. Mirzaei, M. Tanaka, Organometallics 2000, 19, 722.
- 4L. Shi, W. Wang, Y. Wang, Y.-Z. Huang, J. Org. Chem. 1989, 54, 2028.
- 5
- 5aZ.-L. Zhou, L.-L. Shi, Y.-Z. Huang, Tetrahedron Lett. 1990, 31, 7657;
- 5bY.-Z. Huang, Y. Tang, Z.-L. Zhou, W. Xia, L.-P. Shi, J. Chem. Soc. Perkin Trans. 1 1994, 893;
- 5cV. K. Aggarwal, E. Alonso, G. Hynd, K. M. Lydon, M. J. Palmer, M. Porcelloni, J. R. Studley, Angew. Chem. 2001, 113, 1479;
Angew. Chem. Int. Ed. 2001, 40, 1430;
10.1002/1521-3773(20010417)40:8<1430::AID-ANIE1430>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 5dV. K. Aggarwal, E. Alonso, G. Fang, M. Ferrara, G. Hynd, M. Porcelloni, Angew. Chem. 2001, 113, 1482;
Angew. Chem. Int. Ed. 2001, 40, 1433;
10.1002/1521-3773(20010417)40:8<1433::AID-ANIE1433>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 5eZ.-Z. Huang, S. Ye, W. Xia, Y.-H. Yu, Y. Tang, J. Org. Chem. 2002, 67, 3096.
- 6V. K. Aggarwal, Synlett 1998, 329.
- 7For recent reports on phosphane-catalyzed reactions, see:
- 7aM. Wende, R. Meier, J. A. Gladysz, J. Am. Chem. Soc. 2001, 123, 11 490;
- 7bS. A. Frank, D. J. Mergott, W. R. Roush, J. Am. Chem. Soc. 2002, 124, 2404;
- 7cL.-C. Wang, A. L. Luis, K. Agapiou, H.-Y. Jang, M. J. Krische, J. Am. Chem. Soc. 2002, 124, 2402;
- 7dM. Lumbierres, M. Moreno-Mañas, A. Vallribera, Tetrahedron 2002, 58, 4061.
- 8aG. Büchi, H. Wüest, Helv. Chim. Acta 1971, 54, 1767;
- 8bF. Bohlmann, C. Zdero, Chem. Ber. 1973, 106, 3779;
- 8cW. G. Dauben, J. Ipaktschi, J. Am. Chem. Soc. 1973, 95, 5088;
- 8dA. Padwa, L. Brodsky, J. Org. Chem. 1974, 39, 1318;
- 8eD. F. Schneider, A. C. Venter, Synth. Commun. 1999, 29, 1303;
- 8fW. G. Dauben, D. J. Hart, J. Ipaktschi, A. P. Kozikowski, Tetrahedron Lett. 1973, 4425;
- 8gW. G. Dauben, A. P. Kozikowski, Tetrahedron Lett. 1973, 3711;
- 9
- 9aX. Lu, C. Zhang, Z. Xu, Acc. Chem. Res. 2001, 34, 535;
- 9bB. M. Trost, U. Kazmaier, J. Am. Chem. Soc. 1992, 114, 7933;
- 9cC. Guo, X. Lu, J. Chem. Soc. Perkin Trans. 1 1993, 1921;
- 9dJ. Inanaga, Y. Baba, T. Hanamoto, Chem. Lett. 1993, 241;
- 9eB. M. Trost, C.-J. Li, J. Am. Chem. Soc. 1994, 116, 3167;
- 9fB. M. Trost, C.-J. Li, J. Am. Chem. Soc. 1994, 116, 10 819;
- 9gC. Zhang, X. Lu, J. Org. Chem. 1995, 60, 2906;
- 9hB. M. Trost, G. R. Dake, J. Am. Chem. Soc. 1997, 119, 7595;
- 9iB. M. Trost, G. R. Dake, J. Org. Chem. 1997, 62, 5670;
- 9jZ. Xu, X. Lu, J. Org. Chem. 1998, 63, 5031.
- 10Experimental procedures and characterization data of new compounds as well as NOESY spectra of compound 3 c and 1H–1H COSY spectra of compounds 6, 8 a, 9 a, and 11 a are available in the Supporting Information.
- 11The high regioselectivity of the reaction might be explained by steric factors. A similarly high regioselectivity was reported in a phosphane-catalyzed cycloaddition reaction: G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao, X. Zhang, J. Am. Chem. Soc. 1997, 119, 3836.
- 12The isolation of the same product 3 b starting from 4 b and 12 b implies that the reaction of 12 b was initiated by the nucleophilic addition of PPh3 to the electron-deficient double bond of 12 b followed by elimination of the acetate ion (compare entries 3 and 13 of Table 1). The ylide formed from the resulting phosphonium salt is the same as that generated from the reaction of 4 b with PPh3. For a similar addition–elimination reaction, see: M. Harre, P. Raddatz, R. Walenta, E. Winterfeldt, Angew. Chem. 1982, 94, 496; Angew. Chem. Int. Ed. Engl. 1982, 21, 480; D. Seebach, M. Missbach, G. Calderari, M. Eberle, J. Am. Chem. Soc. 1990, 112, 7625.
- 13In general, it is very difficult to generate the ylide directly from allylic acetate without the assistance of a transition metal. For an example of the transition-metal-assisted formation of ylide from allylic acetate, see: R. Tamura, M. Kato, K. Saegusa, M. Kakihana, D. Oda, J. Org. Chem. 1987, 52, 4121.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.