An Unexpected, Sterically Driven, Methyl Halide Elimination in Pentacoordinate Siliconium Halide Salts: Silicon Complexes with Equatorial Nitrogen Coordination†
Daniel Kost Prof.
Department of Chemistry, Ben-Gurion University, Beer-Sheva 84105, Israel, Fax: (+972) 8-647-2943
Search for more papers by this authorBoris Gostevskii Dr.
Ben-Gurion University and, A. E. Favorsky Irkutsk Institute of Chemistry, Russian Academy of Sciences, Russia
Search for more papers by this authorNikolaus Kocher
Institut für Anorganische Chemie der Universität Würzburg,, Germany
Search for more papers by this authorDietmar Stalke Prof. Dr.
Institut für Anorganische Chemie der Universität Würzburg,, Germany
Search for more papers by this authorInna Kalikhman Dr.
Department of Chemistry, Ben-Gurion University, Beer-Sheva 84105, Israel, Fax: (+972) 8-647-2943
Search for more papers by this authorDaniel Kost Prof.
Department of Chemistry, Ben-Gurion University, Beer-Sheva 84105, Israel, Fax: (+972) 8-647-2943
Search for more papers by this authorBoris Gostevskii Dr.
Ben-Gurion University and, A. E. Favorsky Irkutsk Institute of Chemistry, Russian Academy of Sciences, Russia
Search for more papers by this authorNikolaus Kocher
Institut für Anorganische Chemie der Universität Würzburg,, Germany
Search for more papers by this authorDietmar Stalke Prof. Dr.
Institut für Anorganische Chemie der Universität Würzburg,, Germany
Search for more papers by this authorInna Kalikhman Dr.
Department of Chemistry, Ben-Gurion University, Beer-Sheva 84105, Israel, Fax: (+972) 8-647-2943
Search for more papers by this authorDonor-stabilized silyl cations, part 6. For parts 1–5 see reference [6]. This work was supported by the German Israeli Scientific R&D Foundation (GIF), grant I-628-58.5/1999.
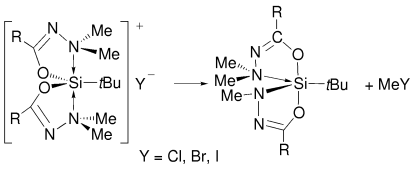
Graphical Abstract
Sterisch gehinderte pentakoordinierte tert-Butylsiliconium-Halogenid-Komplexe gehen Methylhalogenid-Eliminierungen ein. Die Produkte haben eine kovalente und eine koordinative N-Si-Bindung, beide in der äquatorialen Position. Eine vollständige Pseudorotationskoordinate wird anhand geeigneter Strukturen im Kristall dargestellt.
References
- 1For reviews, see:
- 1aP. Lickiss in The Chemistry of Organic Silicon Compounds, Vol. 2, Part 1 (Ed ), Wiley, Chichester, UK, 1998, pp. 557–594;
10.1002/0470857250.ch11 Google Scholar
- 1bJ. B. Lambert, L. Kania, S. Zhang, Chem. Rev. 1995, 95, 1191.
- 2
- 2aJ. Belzner, D. Schär, B. O. Kneisel, R. Herbst-Irmer, Organometallics 1995, 14, 1840;
- 2bD. Schär, J. Belzner in Organosilicon Chemistry III (Ed ), VCH, Weinheim, 1997, p. 429.
10.1002/9783527619900.ch72 Google Scholar
- 3U.-H. Berlekamp, P. Jutzi, A. Mix, B. Neumann, H.-G. Stammler, W. W. Schoeller, Angew. Chem. 1999, 111, 2071;
10.1002/(SICI)1521-3757(19990712)111:13/14<2071::AID-ANGE2071>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2048.10.1002/(SICI)1521-3773(19990712)38:13/14<2048::AID-ANIE2048>3.0.CO;2-C CAS Web of Science® Google Scholar
- 4aC. Chuit, R. J. P. Corriu, A. Mehdi, C. Reyé, Angew. Chem. 1993, 105, 1370;
10.1002/ange.19931050916 Google ScholarAngew. Chem. Int. Ed. Engl. 1993, 32, 1311;
- 4bM. Chauhan, C. Chuit, R. J. P. Corriu, C. Reyé, Tetrahedron Lett. 1996, 37, 845;
- 4cM. Chauhan, C. Chuit, R. J. P. Corriu, A. Mehdi, C. Reyé, Organometallics 1996, 15, 4326.
- 5Yu. E. Ovchinnikov, S. A. Pogozhikh, I. V. Razumovskaya, A. G. Shipov, E. P. Kramarova, S. Yu. Bylikin, V. V. Negrebetsky, Yu. I. Baukov, Russ. Chem. Bull. 1998, 47, 967.
- 6
- 6aI. Kalikhman, S. Krivonos, L. Lameyer, D. Stalke, D. Kost, Organometallics 2001, 20, 1053;
- 6bV. Kingston, B. Gostevskii, I. Kalikhman, D. Kost, Chem. Commun. 2001, 1272;
- 6cD. Kost, V. Kingston, B. Gostevskii, A. Ellern, D. Stalke, B. Walfort, I. Kalikhman, Organometallics 2002, 21, 2293;
- 6dI. Kalikhman, B. Gostevskii, O. Girshberg, S. Krivonos, D. Kost, Organometallics 2002, 21, 2551;
- 6eI. Kalikhman, V. Kingston, B. Gostevskii, V. Pestunovich, D. Stalke, B. Walfort, D. Kost, Organometallics 2002, 21, 4468.
- 7M. Tanaka, Y. Hatanaka, 35th Organosilicon Symposium, Guanajuato, Mexico, 2002, Abstract C-06/IL, p. 28.
- 8For recent reviews on hypervalent silicon complexes, see:
- 8aD. Kost, I. Kalikhman in The Chemistry of Organic Silicon Compounds, Vol. 2, Part 2 (Ed ), Wiley, New York, 1998, p. 1339;
10.1002/0470857250.ch23 Google Scholar
- 8bC. Chuit, R. J. P. Corriu, C. Reyé in The Chemistry of Hypervalent Compounds (Ed.: ), Wiley-VCH, Weinheim, 1999, p. 81;
- 8cBrook, M. A. Silicon in Organometallic and Polymer Chemistry, Wiley, New York, 2000, p. 97.
- 9Elimination of methyl chloride from quaternary hydrazonium chloride compounds (in the absence of silicon coordination) has been reported, and was observed at substantially higher temperature (220 °C): W. Sucrow, M. Slopianka, Chem. Ber. 1978, 111, 780.
- 106 a: T=100(2) K, triclinic, space group: P
 ; a=8.0805(4), b=11.0395(6); c=11.6194(6) Å; α=83.063(1); β=75.121(1); γ=81.860(1)°, Z=2, V=987.79(9) Å3. RF=0.0269 (wRF=0.0737) for I>2σ. 7 b: T=100(2) K, triclinic, space group: P
; a=8.0805(4), b=11.0395(6); c=11.6194(6) Å; α=83.063(1); β=75.121(1); γ=81.860(1)°, Z=2, V=987.79(9) Å3. RF=0.0269 (wRF=0.0737) for I>2σ. 7 b: T=100(2) K, triclinic, space group: P ; a=7.5031(5), b=11.7898(8); c=12.0470(8) Å; α=82.708(1); β=87.980(1); γ=84.512(1)°, Z=2, V=1051.93(12) Å3. RF=0.0345 (wRF=0.0906) for I>2σ. CCDC-195084 (6 a) and CCDC-195085 (7 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
; a=7.5031(5), b=11.7898(8); c=12.0470(8) Å; α=82.708(1); β=87.980(1); γ=84.512(1)°, Z=2, V=1051.93(12) Å3. RF=0.0345 (wRF=0.0906) for I>2σ. CCDC-195084 (6 a) and CCDC-195085 (7 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 11The exact precursors to 7 b (3 b–5 b) did not yield suitable crystals for X-ray crystal-structure analysis. However, the identical 1H, 13C, and 29Si NMR spectra of 3 b and 6 b, as well as previous experience with siliconium salts with different counterions,[6c] indicate that the siliconium ion portions of 3 b and 6 b are equivalent. The replacement of the remote substituent R=tBu in 6 b by R=Me in 6 a is not expected to have a substantial effect on the geometry about the silicon atom.
- 12R. S. Berry, J. Chem. Phys. 1960, 32, 933; R. R. Holmes, J. A. Deiters, J. Am. Chem. Soc. 1977, 99, 3318.
- 13H. B. Bürgi, J. Dunitz, Acc. Chem. Res. 1983, 16, 153.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.